All Blogs
Oct 24, 2019
Rapt Therapeutics files IPO; Novartis stakes $80M for NASH
Rapt Therapeutics files for IPO at $75M Rapt Therapeutics, based in the US, filed to raise to $75 million in its IPO. This had come after five months when the company had retitled itself, which was earlier known as Flx Bio and after three months, it had filed its initial S-1 form with the hope to gain $86 millio...
Read More...
Oct 23, 2019
Biogen seeks the FDA approval to revive its Alzheimer’s drug Aducanumab
Not a long time ago this year, Biogen announced the halting of Phase III clinical trials, EMERGE and ENAGE, of its most looked upon drug aducanumab under simulations for the treatment of Alzheimer's disease called futility analysis. The drug was designed to help patients struggling with mild cognitive impairmen...
Read More...
Oct 22, 2019
FDA approval to AZ’s Farxiga for heart failure; GSK divests two travel vaccines to Bavarian Nordic; New breakthrough therapy for Cystic Fibrosis
The US FDA today has given its nod to Trikafta (elexacaftor/ivacaftor/tezacaftor) of Vertex Pharmaceuticals, the first of its kind triple combination therapy for the patients with cystic fibrosis. The drug, Trikafta, has been approved for patients of age 12 years and older who have at least one F508del mutation...
Read More...
Oct 21, 2019
Emerging drugs bring a positive shift in Global Osteoporosis Market
Osteoporosis is not RARE. As per the International Osteoporosis Foundation (IOF), Osteoporosis affects an estimated 75 million people in Europe, USA and Japan. Approximately 10 million Americans are living with Osteoporosis and around 44 Million are struggling due to low bone density. Osteoporosis is k...
Read More...
Oct 24, 2019
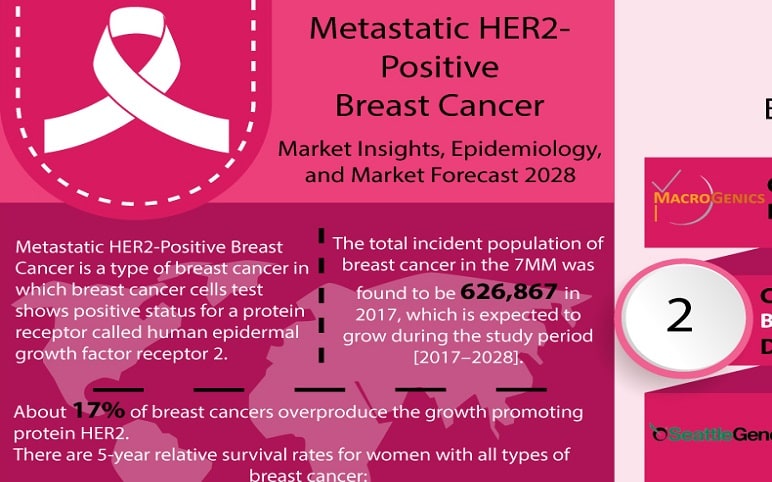
Metastatic HER2-Positive Breast Cancer Infographic
Metastatic HER2-Positive Breast Cancer Metastatic HER2-Positive Breast Cancer is one of the types of breast cancer in which breast cancer cells test show positive status for a protein receptor called human epidermal growth factor receptor 2. The normal function of this protein is to control how a healthy breast...
Read More...
Oct 18, 2019
Incyte Corporation submits positive results of trials for its drug Ruxolitinib in GvHD
Incyte Corporation has recently announced the results of Novartis-sponsored Phase III clinical trials REACH2 evaluating its candidate Ruxolitinib (Jakafi) in patients suffering from steroid-refractory acute graft-versus-host disease (GvHD). Graft versus host disease (GvHD), as defined by the National Cancer Institu...
Read More...
Oct 17, 2019
EicOsis receives USD 15M to fuel its non-opioid pain therapy; Alexion Pharmaceuticals buys Achillion Pharmaceuticals; Ipsen adds another drug to its rare disease pipeline
EicOsis has received USD 15Million grant from the National Institute of Drug Abuse (NIDA) to fund the clinical trials of non-opioid pain therapy. NIDA, with an aim to supplement NIH’s initiative Helping to End Addiction Long-Term (HEAL Initiative), has awarded the grant to the company. Deaths due to drug ov...
Read More...
Oct 16, 2019
New Therapeutic advances that have shifted the Breast cancer market scenario
HR-positive/HER2-negative breast cancer treatment landscape focuses on the complexity of the specific cancer type and the treatment differ according to the biomarker status. The trend suggests a greater bend towards personalized medicine based upon patient biomarkers and physiologic characteristics In our last a...
Read More...
Oct 16, 2019
Down Syndrome Awareness Month
Down Syndrome Awareness Month Every year, October is observed as World Down Syndrome awareness month, aiming to aware the general population about Down syndrome. It is a genetic disorder, also known as Trisomy 21 that is caused by the presence of partial or complete of a third copy of a chromosome 21. The early ...
Read More...
Oct 20, 2019
World Osteoporosis Day
World OSTEOPOROSIS day, observed on 20th October every year to raise awareness about diagnosis, treatment, protection and prevention of Osteoporosis. This condition develops when bone density increases, the body reabsorbs more of the bone tissue and produces less amount to replace it. Changes in the lifestyle ...
Read More...