All Blogs
Aug 16, 2024
YORVIPATH for Hypoparathyroidism Treatment – The First and Only Therapy for Therapy for Adults
A year after rejecting it, the FDA has now approved Ascendis Pharma’s once-daily injectable YORVIPATH for hypoparathyroidism treatment. Ascendis Pharma’s YORVIPATH (palopegteriparatide), formerly called TransCon PTH, has been approved for the treatment of adults with hypoparathyroidism. This rare condition, charact...
Read More...
Aug 14, 2024
7 HSV-associated Oncolytic Virus Therapies in Late and Mid-Stage
Herpes Simplex Virus (HSV)-associated oncolytic virus therapeutics are a promising approach to cancer treatment. These treatments use genetically engineered HSV to target and destroy cancer cells while preserving healthy tissue specifically. The HSV is designed to increase selectively within tumor cells, causing th...
Read More...
Aug 13, 2024
FDA Approves Ascendis Pharma’s YORVIPATH; ARS Pharma Gets FDA Green Light for First Nasal Spray; FDA Rejects Lykos’ MDMA-Assisted PTSD Therapy; Novartis’ FABHALTA Receives FDA Accelerated Approval; Servier Snags FDA Approval for Voranigo
Ascendis Pharma Lands Long-Awaited FDA Drug Approval in Rare Hormone Deficiency Ascendis Pharma A/S has announced that the FDA has approved YORVIPATH (palopegteriparatide; developed as TransCon PTH) for the treatment of hypoparathyroidism in adults. YORVIPATH, a prodrug of parathyroid hormone (PTH[1-34]), is adm...
Read More...
Aug 12, 2024
TECELRA Approval for Synovial Sarcoma Treatment – A Ray of Hope
Adaptimmune Therapeutics and patients waiting for synovial sarcoma treatment witnessed a historic moment at the start of this month. After over ten years without new synovial sarcoma treatments, a groundbreaking, first-in-class cell therapy, TECELRA (afamitresgene autoleucel) has been approved by the FDA. This a...
Read More...
May 12, 2025
9 Promising Obesity Drugs Set to Launch by 2030
The pharmaceutical industry is witnessing a seismic shift, fueled by the meteoric rise of GLP-1 agonist molecules. Once a niche class of diabetes drugs, GLP-1 agonists have become the cornerstone of a global race to conquer obesity, a disease that has more than doubled in prevalence since 1990 and now affects over ...
Read More...
Aug 08, 2024
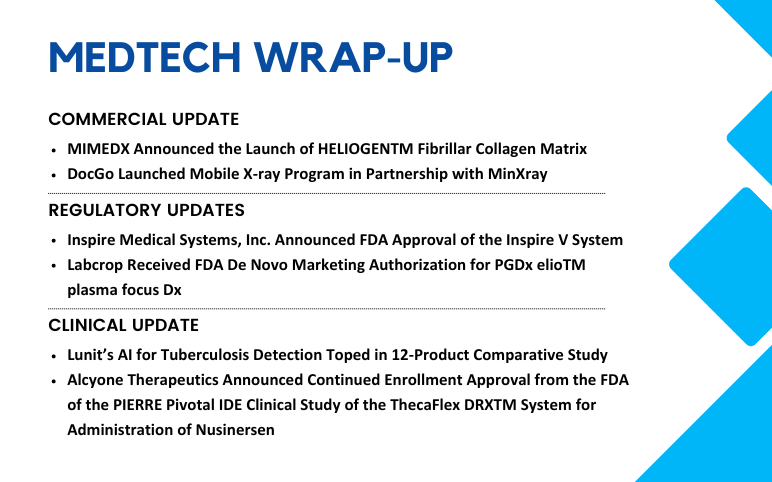
MIMEDX Launches HELIOGENTM Fibrillar Collagen Matrix; DocGo Launches Mobile X-ray Program; Labcrop’s FDA De Novo Marketing Authorization; Inspire Medical Systems Receives FDA Approval; Alcyone Therapeutics Announces Continued Enrollment Approval from the FDA; Lunit’s AI for Tuberculosis Detection
MIMEDX Announced the Launch of HELIOGENTM Fibrillar Collagen Matrix On July 31, 2024, MiMedx Group, Inc. launched HELIOGEN™ Fibrillar Collagen Matrix, a particulate xenograft product designed to treat complex wounds, especially in surgical environments. HELIOGEN is a shelf-stable product that contains Type...
Read More...
Aug 07, 2024
8 Late and Mid Stages Adenovirus-associated Oncolytic Virus Therapies To Watch Out
Adenovirus-associated oncolytic virus therapies represent a promising approach to cancer treatment by harnessing the inherent ability of viruses to selectively infect and destroy cancer cells. These therapies utilize genetically modified adenoviruses, which are engineered to target and replicate specifically within...
Read More...
Aug 06, 2024
Tecelra by Adaptimmune: First FDA-Approved Engineered Cell Therapy for Solid Tumors; GSK’s JEMPERLI Approved for Endometrial Cancer; MBX Biosciences Secures $63.5M in Series C for PEP™ Platform; FDA Stops Actinium’s Radiotherapy Blood Cancer Plans, Pushing Partnering Shift; Otsuka Acquires Jnana for Up to $1.1B, Enhancing Drug Discovery
Adaptimmune’s TECELRA Becomes First FDA-Approved Engineered Cell Therapy for Solid Tumors Adaptimmune Therapeutics announced that the FDA has approved (afamitresgene autoleucel), which will be marketed under the brand name TECELRA, for the treatment of unresectable or metastatic synovial sarcoma. This marks the ...
Read More...
Aug 05, 2024
DARZALEX FASPRO Regimen Approval: Johnson & Johnson Howling Success in Multiple Myeloma
Despite significant progress in multiple myeloma treatment with a range of biological drugs and their combinations—such as IMiDs (BMS REVLIMID, POMALYST/IMNOVID), anti-CD38 monoclonal antibody drugs (DARZALEX, SARCLISA), the anti-SLAM7 monoclonal antibody (AbbVie and BMS multiple myeloma drug EMPLICITI), and new pr...
Read More...
Mar 12, 2025
WEGOVY: A Leader in the Obesity and Weight Loss Treatment
With the growing global health concern regarding obesity, Novo’s WEGOVY (semaglutide) has emerged as a leading solution in the weight loss treatment market. WEGOVY is a GLP-1 receptor agonist used alongside a low-calorie diet and increased physical activity. This obesity medication is administered as a weekly subcu...
Read More...