All Blogs
Aug 29, 2024
Insulet Corporation’s Omnipod® 5 Automated Insulin Delivery System Receives FDA Approval; FDA Gives Green Light to Expand Vericel’s MACI Arthro Label; SkinCure Oncology Announces New Study of Non-melanoma Skin Cancer Through Image-guided Superficial Radiation Therapy; SDC’s BYOD ePRO Solution Clinical Trial; PaceMate® Acquires Paceart Optima™ System; KARL STORZ Acquires a US-headquartered Company
FDA Cleared the Omnipod® 5 Automated Insulin Delivery System for Use in People with Type 2 Diabetes On August 26, 2024, Insulet Corporation, the global leader in tubeless insulin pump technology with its Omnipod® brand of products, announced that its groundbreaking Omnipod 5 Automated Insulin Delivery Syst...
Read More...
Aug 28, 2024
Top 7 Emerging Vaccinia Virus-associated Oncolytic Virus Therapies
Vaccinia virus-associated oncolytic virus therapies represent a promising approach to cancer treatment, leveraging the ability of the vaccinia virus to selectively infect and destroy cancer cells. The vaccinia virus, historically used in the smallpox vaccine, has been engineered to serve as an oncolytic virus due t...
Read More...
Aug 27, 2024
Merck’s WINREVAIR™ EU Approval; BALVERSA for Urothelial Carcinoma; Novartis and Versant’s Borealis Launched; Moderna’s RSV mRESVIA® EU Approval; FDA Nods RYBREVANT® and LAZCLUZE™ for EGFR-mutated Lung Cancer
Merck’s WINREVAIR Approved by the European Commission for PAH in Adults with Functional Class II-III Merck has secured European Commission (EC) approval for WINREVAIR™ (sotatercept), marking it as the first activin signaling inhibitor therapy for pulmonary arterial hypertension (PAH) approved across all 27 EU me...
Read More...
Aug 26, 2024
Imbalance Between BCG Supply and Increased Demand: The Crisis Impacting Bladder Cancer Treatment
The BCG vaccine, a significant medical breakthrough developed in the early 20th century, is derived from a strain of Mycobacterium bovis initially created by the University of Illinois as a vaccine against tuberculosis. For NMIBC, BCG is administered intravesically to reduce the risk of cancer recurrence and progre...
Read More...
Aug 23, 2024
Exploring Growth Opportunities in the Hypophosphatasia Treatment Market: Navigating the Ultra-rare Disease Landscape
Quick Facts According to DelveInsight’s analysis, in 2023, the total diagnosed prevalent cases of hypophosphatasia, an ultra-rare disease, was approximately 6,200 in the 7MM. While almost 85% have less severe forms of the disease, severity may vary based on genetic and environmental factors. Due to the low n...
Read More...
Aug 22, 2024
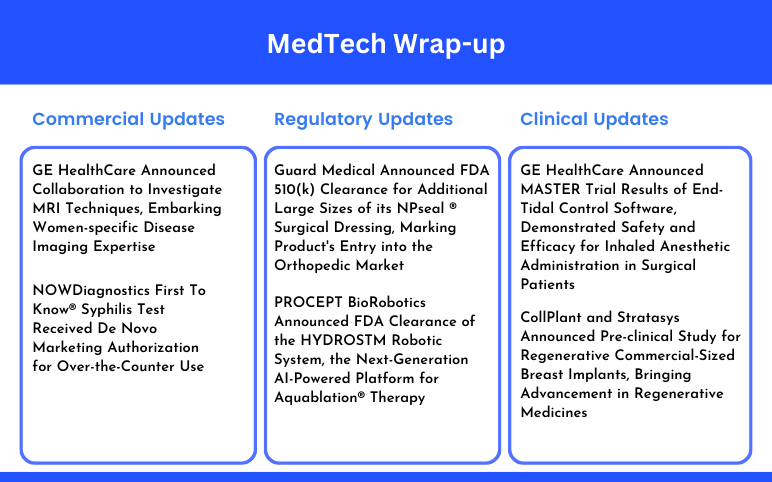
Guard Medical Announced FDA 510(k) Clearance; PROCEPT BioRobotics Announced FDA Clearance; GE HealthCare Announced MASTER Trial Results; CollPlant and Stratasys Announced Pre-clinical Study; GE HealthCare Announced Collaboration to Investigate MRI Techniques; NOWDiagnostics’ Syphilis Test Received De Novo Marketing Authorization
Guard Medical Announced FDA 510(k) Clearance for Additional Large Sizes of its NPseal ® Surgical Dressing, Marking Product's Entry into the Orthopedic Market On August 19, 2024, Guard Medical, Inc., a medical technology company, announced FDA 510(k) clearance for expanded large sizes (up to 25cm) of its advanced...
Read More...
Mar 12, 2025
8 Emerging Obesity Trends Transforming Therapeutics Segment
Obesity is the second leading cause of preventable death and is linked to numerous inflammatory conditions, which contribute to diseases like cardiovascular disease, diabetes, respiratory issues, mental health disorders, hypertension, obstructive sleep apnea, cancer, and high cholesterol. This poses a significant p...
Read More...
Aug 20, 2024
Gilead’s Livdelzi FDA Approval for Primary Biliary Cholangitis; Incyte and Syndax’s Niktimvo Approved for Graft-Versus-Host Disease; FDA Lifts Hold on BioNTech and MediLink’s ADC Cancer Trial; FDA Approves IMFINZI for Resectable Lung Cancer; Yutrepia Receives Tentative Approval for PAH and PH-ILD
Gilead’s Livdelzi (Seladelpar) Granted Accelerated Approval for Primary Biliary Cholangitis by FDA Gilead Sciences, Inc. has received accelerated approval from the FDA for Livdelzi® (seladelpar) in the treatment of primary biliary cholangitis (PBC). Livdelzi can be used in combination with ursodeoxycholic acid (...
Read More...
Aug 19, 2024
Novartis’ FABHALTA: Leading the Way as the First Complement Inhibitor for IgAN
The first horse bearing Novartis’ IgAN treatment has reached the FDA’s approval milestone. FABHALTA is approved for use in treating IgAN patients who are at risk of swift disease progression, as evidenced by a urine protein-to-creatinine ratio of 1.5 g/g or higher. On August 8, the FDA approved the use of Novart...
Read More...
Aug 15, 2024
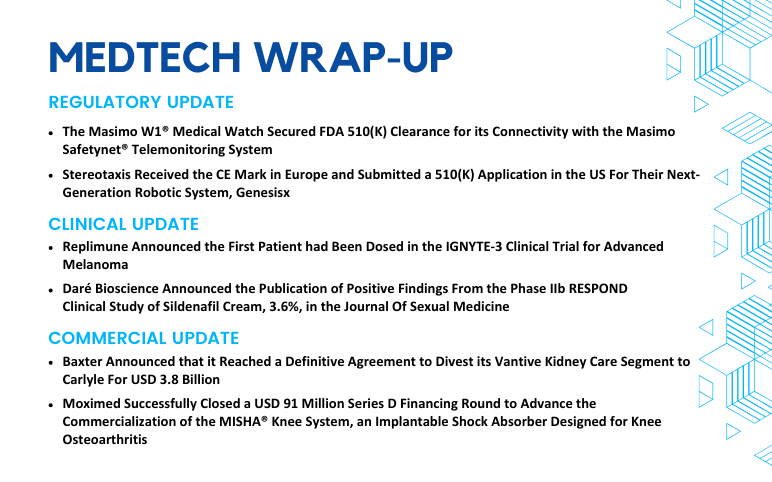
Masimo W1® Medical Watch Secured FDA 510(K) Clearance; Stereotaxis Received the CE Mark in Europe and Submitted a 510(K) Application in the US; Replimune Dosed First Patient in the IGNYTE-3 Clinical Trial; Daré Bioscience Announced the Publication of Positive Findings From the Phase IIb RESPOND Clinical Study; Baxter’s Definitive Agreement to Carlyle For USD 3.8 Billion; Moximed Successfully Closed a USD 91 Million Series D Financing Round
The Masimo W1® Medical Watch Secured FDA 510(K) Clearance for its Connectivity with the Masimo Safetynet® Telemonitoring System On August 12, 2024, Masimo announced that its Masimo W1® medical watch received FDA 510(k) clearance for connectivity. This approval enabled the watch to integrate with the Masimo...
Read More...