All Blogs
Jul 18, 2024
Inspira™ Approval of INSPIRA™ ART100 System; Oticon Medical’s Sentio™ System Received Regulatory Clearance; SpineGuard Filed its “510K” Dossier in the US; Vectorious Medical Technologies’ V-LAP Left Atrial Pressure Sensor Successful Implantation; Bedal International Raised $11 Million; Mytonomy Inc. Agreement With Vizient, Inc.
Inspira™ Secured Approval From Israeli Authorities for its INSPIRA™ ART100 System On July 11, 2024, Inspira™ Technologies OXY B.H.N. Ltd., a pioneering medical technology company, secured a pivotal milestone with the receipt of the Israeli Ministry of Health's medical devices and accessories ("AMAR") approval fo...
Read More...
Jul 17, 2024
Vaccine Adjuvants: Enhancing Immunity in the Fight Against Diseases
Vaccines have been one of the most significant advancements in medical science, saving millions of lives by preventing infectious diseases. While the primary component of a vaccine is the antigen that stimulates an immune response, another crucial element often goes unnoticed: the adjuvant. What are Vaccin...
Read More...
Jul 16, 2024
Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipron; FDA Issues Complete Response Letter to Novo Nordisk; Arcutis’ ZORYVE® Cream 0.15% FDA Approval; NICE Recommends Ebglyss For Moderate To Severe Atopic Dermatitis
Immutep Announces Promising Outcomes for First-Line Treatment in PD-L1 Negative Head and Neck Squamous Cell Carcinoma Patients Immutep Limited announced positive results from Cohort B of the TACTI-003 (KEYNOTE-PNC-34) Phase IIb trial, evaluating eftilagimod alfa (efti) combined with MSD’s anti-PD-1 therapy KEYTR...
Read More...
Jul 15, 2024
FcRn Inhibitors Being The Fastest Growing Class, Plans To Get Explored In 20+ Indications
Vyvgart enjoying strong commercial uptake whereas RYSTIGGO is preferred for MuSK+ Myasthenia gravis. What do the physicians believe? The neonatal Fc receptor (FcRn) inhibitor market is heating up, with a growing cast of leading contenders such as Argenx, JnJ, and UCB pharma, along with a few early-stage players ...
Read More...
Jul 12, 2024
Eli Lilly strengthening its Gastroenterology portfolio post Acquisition of Morphic. Tracking competitors and alliances in inflammatory bowel disease (IBD).
In a strategic move aimed at bolstering its portfolio in inflammatory bowel disease (IBD) treatments, Eli Lilly and Company has announced a definitive agreement to acquire Morphic Holding for approximately USD 3.2 billion. The purchase price represents a 79% premium to Morphic’s closing stock price. Post the tender...
Read More...
May 08, 2025
Top 7 Breakthrough Drugs for Ulcerative Colitis Treatment
Ulcerative colitis is a type of inflammatory bowel disease of unknown origin that targets the lining of the colon. Symptoms typically include diarrhea, abdominal pain, discomfort, and the presence of blood in the stool. The severity of the condition can vary, with inflammation affecting just the rectum, extending t...
Read More...
Jul 11, 2024
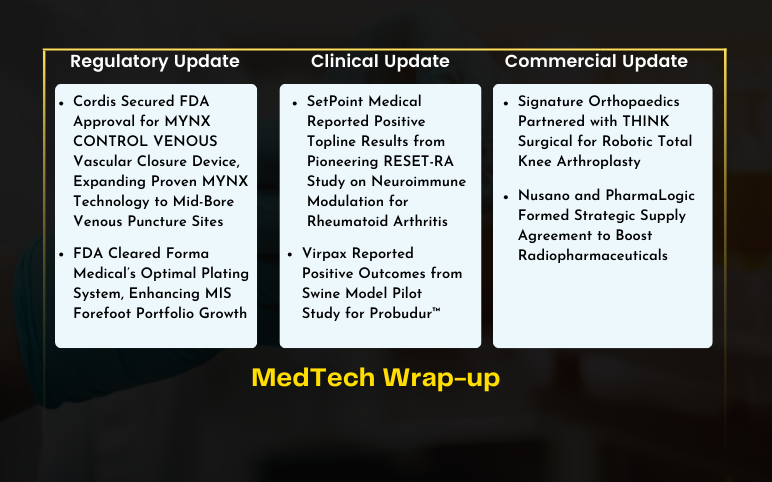
Cordis FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device; Forma Medical’s Optimal Plating System FDA Approval; Virpax’s Swine Model Pilot Study Positive Results; SetPoint Medical Positive Topline Results from Pioneering RESET-RA Study; Signature Orthopaedics Partnered with THINK Surgical; Nusano and PharmaLogic Formed Strategic Supply Agreement
Cordis Secured FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device, Expanding Proven MYNX Technology to Mid-Bore Venous Puncture Sites On July 9, 2024, Cordis, a leading company in cardiovascular and endovascular technology, received FDA approval for its MYNX CONTROL™ VENOUS Vascular Closure Device. Thi...
Read More...
Jul 10, 2024
Gummy Vitamins: A Sweet Solution to Nutrient Deficiencies?
In recent years, gummy vitamins have surged in popularity, becoming a staple in many households. Originally designed for children, these chewable, sweet, and often colorful supplements are now widely consumed by adults as well. Their popularity is not just a trend; it's a reflection of their convenience, taste, and...
Read More...
Jul 09, 2024
Lilly’s Morphic Acquisition; IDEAYA’s IDE397 Positive Phase II Trial Result; XPOVIO (selinexor) Approval in China; Roche to Reintroduce Susvimo in the US; Dupixent EU Approval
Lilly Strengthens IBD Treatment Portfolio with Morphic Acquisition Eli Lilly and Company and Morphic Holding, Inc. announced a definitive agreement for Lilly to acquire Morphic, a biopharmaceutical company developing oral integrin therapies for serious chronic diseases. Lilly will initiate a tender offer to acqu...
Read More...
Jul 08, 2024
A New Dawn in Alzheimer’s Disease Treatment: Eli Lilly’s Donanemab Wins FDA Approval
The world of Alzheimer’s disease treatment witnessed a groundbreaking moment recently as the FDA approved Eli Lilly’s donanemab, marketed as KISUNLA. This new drug brings hope to millions of patients and their families struggling with this debilitating disease. Let us delve into the fascinating journey of donanemab...
Read More...