All Blogs
Jun 27, 2024
Advancing Diabetes Care: Highlights from the ADA’s 84th Scientific Sessions
The American Diabetes Association (ADA), a premier voluntary health organization, has been at the forefront of combating the diabetes epidemic and enhancing the lives of individuals with diabetes for 83 years. The ADA has consistently fueled discovery and research aimed at treating, managing, and preventing diabete...
Read More...
Jun 25, 2024
AstraZeneca’s AKT Inhibitor TRUQAP Falls Short in Triple Negative Breast Cancer
Triple-negative breast cancer (TNBC) constitutes about 10-15% of all breast cancer cases with nearly 44,000 incident cases in the United States in 2023, as per Delveinsight’s estimates. It is a rare but aggressive form of breast cancer. Treatment for TNBC is more limited than for other breast cancer types. C...
Read More...
Jun 25, 2024
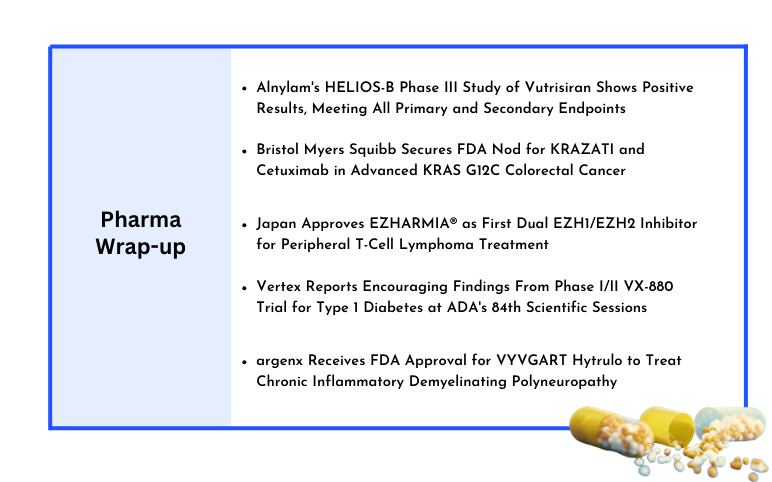
Alnylam’s HELIOS-B Phase III Study of Vutrisiran; Bristol Myers Squibb Secures FDA Nod for KRAZATI and Cetuximab; Daichii Sankyo’s EZHARMIA® Receives Japan Approval; Vertex’s Phase I/II VX-880 Trial; argenx’s VYVGART Hytrulo FDA Approval
Alnylam's HELIOS-B Phase III Study of Vutrisiran Shows Positive Results, Meeting All Primary and Secondary Endpoints Alnylam Pharmaceuticals, Inc. reported encouraging topline outcomes from its HELIOS-B Phase III study of vutrisiran, an experimental RNAi therapy being developed to treat ATTR amyloidosis with car...
Read More...
Jun 24, 2024
Revolutionizing Dilated Cardiomyopathy Treatment: The Road Ahead
Quick facts The primary cause of DCM is understood to be genetic, leading to a focus on tailoring treatments based on the specific molecular origin. Genetic testing plays a crucial role in DCM, as numerous studies and case reports have demonstrated, serving as a vital tool for diagnosis, prognosis, familial scr...
Read More...
Sep 24, 2024
Unveiling Amyotrophic Lateral Sclerosis (ALS) Treatment Frontiers: Navigating Challenges, Overcoming Setbacks, and Emerging Therapeutic Horizons
DelveInsight forecasts that with the launch of 15+ emerging therapies alongside existing treatments, the ALS treatment market is anticipated to surpass USD 4 billion across the 7MM by 2034. Currently, there is no cure for ALS and no effective treatment to halt or reverse the progression of the disease. As per ou...
Read More...
Jun 20, 2024
Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Venous Stent System; Akili’s EndeavorOTC Received FDA Clearance; Roche Achieved FDA Clearance; atHeart Medical Reported Long and Short-term Clinical Data; Bioretec Announced Successful Clinical Outcomes
Boston Scientific Corporation Announced Definitive Agreement to Acquire Silk Road Medical, Inc., an Innovator in Stroke Prevention Technology On June 18, 2024, Boston Scientific Corporation, a global leader in medical technologies, announced the formal agreement to acquire Silk Medical, Inc., a medical device co...
Read More...
Jun 19, 2024
Gene therapy: Following Pfizer’s Unexpected Failure, Sarepta is celebrating its much-awaited full approval! Sarepta’s stock price has shot up over 32%
Duchenne muscular dystrophy (DMD) is a severe genetic disorder primarily affecting young boys between the ages of two and three. As per Delveinsight’s estimates, there were approximately 16K DMD patients in 2023 in the United States, with ˜75% of the patients below the age of 15. This condition leads to progressive...
Read More...
Jun 26, 2024
Understanding Asthma Diagnostic Devices: Enhancing Management and Care
Asthma is a major noncommunicable disease that impacts both children and adults, and it is the most prevalent chronic condition among children. In 2019, ~262 million individuals were impacted by asthma, resulting in 455K deaths all over the world. More than 27 million individuals in the United States suffer from...
Read More...
Jun 18, 2024
Takeda Showcase Phase III Results for Soticlestat; Imfinzi and Chemotherapy Combination Gains US Approval; Nipocalimab Shows Notable Efficacy in Phase II Study; Bristol Myers Squibb’s Augtyro FDA Approval; AstraZeneca’s Farxiga FDA Approval
Takeda Reveals Phase III Results for Soticlestat (TAK-935) in Dravet and Lennox-Gastaut Syndromes Takeda revealed topline results from its SKYLINE and SKYWAY studies. SKYLINE (TAK-935-3001) was a multicenter, randomized, double-blind Phase III trial assessing soticlestat (TAK-935) plus standard care against plac...
Read More...
Jun 17, 2024
WINREVAIR Approval for Pulmonary Arterial Hypertension Treatment: Is It A Game-Changer for Merck?
Merck’s investment of $11.5 billion in acquiring Acceleron Pharma is expected to yield significant returns with the FDA’s approval of WINREVAIR (sotatercept), a pivotal component of the acquisition, for the treatment of pulmonary arterial hypertension. An estimated prevalence of pulmonary hypertension at the pop...
Read More...