All Blogs
Apr 05, 2024
Advancements in Targeting KRASG12D: Preclinical Insights and Therapeutic Potential
KRAS mutations represent the most commonly encountered driver oncogene, implicated in approximately 25% of all human cancers, with KRASG12D emerging as a predominant isoform, particularly prevalent in pancreatic, colorectal, and non-small cell lung cancers. Despite its frequency, targeting KRASG12D poses distinct c...
Read More...
Apr 05, 2024
Vanda Broadens its Reach to Bipolar Disorder Treatment Market With Fanapt Approval
Fifteen years after its first FDA approval for treating schizophrenia with Fanapt (iloperidone), Vanda Pharmaceuticals has achieved another success. Fanapt is a type of antipsychotic medication that deviates from the usual and has been administered to individuals with schizophrenia for their immediate treatment fol...
Read More...
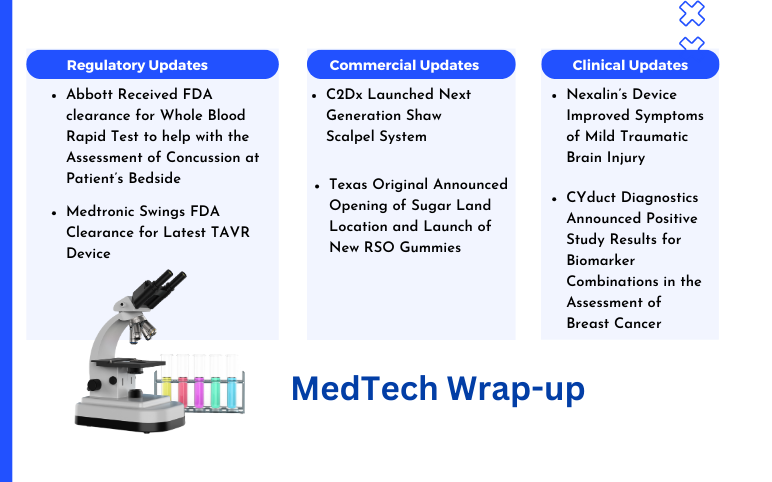
Apr 04, 2024
C2Dx’s Shaw Scalpel System; Texas Original’s RSO Gummies; Abbott’s Whole Blood Rapid Test FDA Clearance; Medtronic’s TAVR Device FDA Clearance; Nexalin’s Device Improved Symptoms; CYduct Diagnostics’ Positive Study Results
C2Dx Launched Next Generation Shaw Scalpel System On March 26, 2024, C2Dx, a privately-owned medical device company, announced the launch of its Next Generation Shaw Scalpel System. The Shaw Scalpel System has obtained 510(k) clearance from the FDA for the upgraded controller of its advanced surgical techn...
Read More...
Apr 03, 2024
Nutrition Nexus: Illuminating the Growth Trajectory Path in the Dietary Supplements Market
The Dietary Supplements market has experienced significant growth over the past years, driven by a variety of factors contributing to its sustained demand. One primary reason for this surge is the increasing awareness and emphasis on preventive healthcare and wellness among consumers worldwide. As people become mor...
Read More...
Apr 02, 2024
AstraZeneca’s Voydeya FDA Approval; Akebia’s Vafseo FDA Approval; Bristol Myers Squibb’s Phase III YELLOWSTONE Trial Update; Astellas’ IZERVAY FDA Approval; AstraZeneca’s Truqap and Faslodex MHLW Approval
Voydeya Receives FDA Approval as Supplemental Treatment with Ravulizumab or Eculizumab for Managing Extravascular Hemolysis in Adult Patients with PNH Voydeya (danicopan) has received approval in the United States for use alongside ravulizumab or eculizumab in treating extravascular hemolysis (EVH) in adults dia...
Read More...
Apr 01, 2024
Advances in Non-Muscle Invasive Bladder Cancer Treatment: Exploring Emerging Therapies
Non-muscle-invasive bladder cancer represents a category of bladder cancer where the tumor is confined to the innermost layer of the bladder lining without invading the muscle. This early-stage form accounts for a significant proportion of bladder cancer cases. Among the 7MM, the US accounted for the highest num...
Read More...
Mar 29, 2024
Potential of EGFR Inhibitors: A Promising Avenue in Cancer Treatment
In the realm of cancer treatment, the development of targeted therapies has been nothing short of revolutionary. Among these advancements, EGFR inhibitors have emerged as a powerful weapon in the fight against certain types of cancer. EGFR, or Epidermal Growth Factor Receptor, is a protein found on the surface of c...
Read More...
Mar 28, 2024
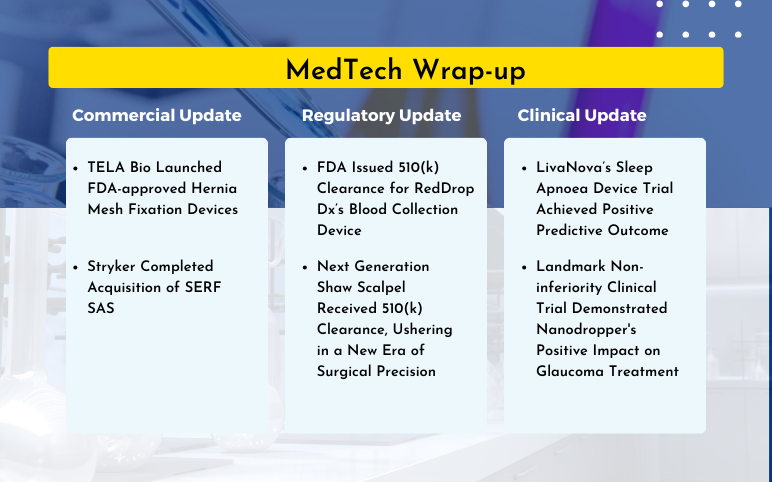
TELA Bio Launched Hernia Mesh Fixation Devices; Stryker Acquisition of SERF SAS; FDA 510(k) Clearance for RedDrop Dx’s Blood Collection Device; FDA 510(k) Clearance for Next Generation Shaw Scalpel; LivaNova’s Sleep Apnoea Device Trial; Nanodropper’s Positive Impact on Glaucoma Treatment
TELA Bio Launched FDA-approved Hernia Mesh Fixation Devices On March 21, 2024, TELA Bio, Inc., a medical technology firm, launched its FDA-approved LIQUIFIX devices for hernia repairs in the United States. The LIQUIFIX FIX8™ and LIQUIFIX Precision™ are specifically engineered for laparoscopic and open femo...
Read More...
Oct 03, 2025
Botanical Bounty: Cracking the Herbal Medicine Market Enigma and Growth Potential
Herbal medicine, once rooted in folk traditions, has evolved into a structured and evidence-based segment of modern healthcare. The global herbal medicine market and herbal medicinal products market are witnessing strong growth, with the US herbal medicine market gaining momentum alongside Asia and Europe’s longsta...
Read More...
Mar 26, 2024
Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabhalta CHMP Approval; Idorsia’s TRYVIO FDA Approval; AbbVie’s Landos Biopharma Acquisition
Regeneron Updates Progress on Biologics License Application for Odronextamab Regeneron Pharmaceuticals, Inc. has announced that the FDA has issued Complete Response Letters (CRLs) regarding the Biologics License Application (BLA) for odronextamab in cases of relapsed/refractory (R/R) follicular lymphoma (FL) and...
Read More...