All Blogs
Feb 27, 2024
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease; GSK Announces Positive Headline Results from EAGLE-1 Phase III Trial; Dupixent sBLA Accepted for FDA Priority Review; Biogen’s QALSODY Received Positive Opinion from CHMP; FDA Granted Orphan Drug Designation to Immune-Onc’s IO-202; Artiva Biotherapeutics’s AlloNK® in Lupus Nephritis
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease due to MASH, with Significant Improvements in Fibrosis Boehringer Ingelheim has reported that in a Phase II trial, a significant proportion of adults treated with survodutide (BI 456906), up to 83.0%, showed a notable enhancement in metabol...
Read More...
Feb 26, 2024
Iovance’s Breakthrough: Amtagvi Paves the Way for Cell Therapies in Solid Tumors
The category of T-cell therapy, which revolutionized the management of specific blood cancers, has now extended its impact to solid tumor treatment with the approval from the FDA of a groundbreaking immunotherapy pioneered by Iovance Biotherapeutics. Known as Amtagvi or lifileucel, this medication marks the debut o...
Read More...
Feb 23, 2024
Ipsen’s Onivyde Combo Ends a Decade-Long Dry Spell in Newly Diagnosed Pancreatic Cancer
It marks the awaited green light for Ipsen following its acquisition of Onivyde in 2017. The FDA has given its nod to the supplemental new drug application for Onivyde (irinotecan liposome injection) in combination with oxaliplatin, fluorouracil, and leucovorin (NALIRIFOX) as a primary treatment for adults with met...
Read More...
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Feb 21, 2024
Hormone Replacement Therapy Market: Unveiling the Growth Opportunities and Key Players Exploring the Domain
Hormone Replacement Therapy (HRT) stands as a big ray of hope for individuals affected by the multifaceted challenges of hormonal imbalances. Defined as the administration of synthetic or natural hormones to supplement or replace the body's own hormonal levels, Hormone Replacement Therapy has emerged as a cornersto...
Read More...
Feb 20, 2024
FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas and Kelonia Enter into Research and License Agreement; Fast Track Designation to Certa’s FT011; Innovent Announces Phase 3 Clinical Trial Updates for IBI311; Orphan Drug Designation to Cardiol’s Pericarditis Drug Candidate
FDA Approves Xolair as First and Only Medicine for Children and Adults with One or More Food Allergies Roche has announced that the FDA has approved Xolair® (omalizumab) to mitigate allergic responses, such as anaphylaxis, that may arise from accidental exposure to various foods in both adult and pediatric patie...
Read More...
Feb 19, 2024
Advancements in Complement 3 Glomerulopathy (C3G) Treatment: Exploring Upcoming Anti-Complements
Complement 3 glomerulopathy (C3G) is a form of glomerular disease where there’s a notable presence of C3 complement component deposits in the glomeruli. These deposits occur without significant amounts of immunoglobulin and without the deposition of C1q and C4. The buildup of C3, without much classical or lectin co...
Read More...
Feb 16, 2024
Will Roche’s Crovalimab An Answer to AstraZeneca PNH Treatment Drugs?
China has become the first nation to approve Roche’s crovalimab, a paroxysmal nocturnal hemoglobinuria (PNH) treatment. Unlike AstraZeneca’s infused treatments Soliris and Ultomiris, crovalimab is administered subcutaneously. Developed by Roche’s subsidiary Chugai Pharmaceutical, crovalimab is a humanized complemen...
Read More...
Feb 15, 2024
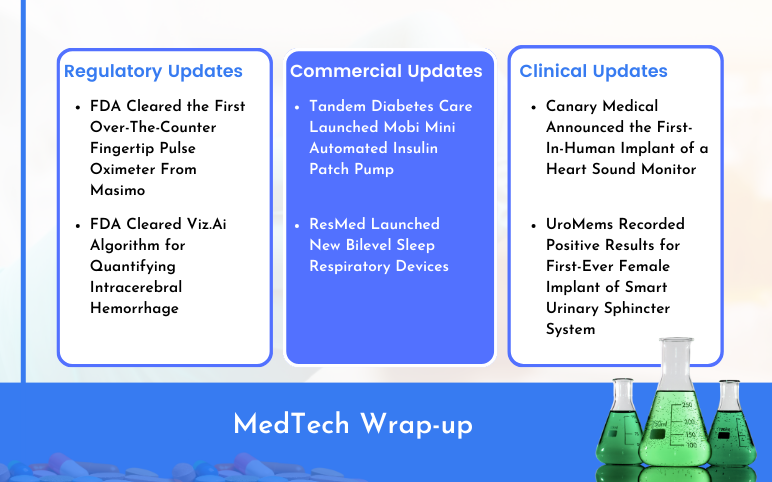
Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First Over-The-Counter Fingertip Pulse Oximeter; Viz.Ai Algorithm for Quantifying Intracerebral Hemorrhage; Canary Medical’s Heart Sound Monitor; UroMems’ Smart Urinary Sphincter System
Tandem Diabetes Care Launched Mobi Mini Automated Insulin Patch Pump On February 13, 2024, announced that it started off the U.S. commercial launch of its Mobi insulin patch pump. The San Diego-based business claims that Mobi, which is fully controllable via a smartphone app, is the world's smallest durable a...
Read More...
Feb 14, 2024
Pressure Relief Devices: Charting the Course of Market Evolution and Major Developments
Pressure relief devices have undergone a remarkable evolution over the years, catering to the diverse needs of individuals for enhanced comfort and medical support. Initially simple in design, these devices have evolved into sophisticated solutions that address specific medical conditions and promote overall well-b...
Read More...