All Blogs
Feb 14, 2024
Pressure Relief Devices: Charting the Course of Market Evolution and Major Developments
Pressure relief devices have undergone a remarkable evolution over the years, catering to the diverse needs of individuals for enhanced comfort and medical support. Initially simple in design, these devices have evolved into sophisticated solutions that address specific medical conditions and promote overall well-b...
Read More...
Feb 13, 2024
GSK Receives FDA Fast Track Designation for Bepirovirsen; Gilead to Acquire CymaBay Therapeutics; CSL Announces Top-line Results from the Phase III AEGIS-II Trial; Ruxoprubart Scores FDA Orphan Drug Designation for PNH Treatment; CymaBay Announces FDA Acceptance of NDA and Priority Review for Seladelpar; Biogen Received European Commission Approval for SKYCLARYS
GSK Receives FDA Fast Track Designation for Bepirovirsen in Chronic Hepatitis B GSK plc has revealed that the US Food and Drug Administration (FDA) has awarded Fast Track status to bepirovirsen, an experimental antisense oligonucleotide (ASO) designed to treat chronic hepatitis B (CHB). Fast Track designation ai...
Read More...
Oct 11, 2024
Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
Glioma and glioblastoma are both types of brain tumors, but they differ significantly in their characteristics and prognosis. Glioma is a broad term that encompasses tumors originating from glial cells, which are supportive cells in the brain. These tumors can vary in grade and aggressiveness, with some growing slo...
Read More...
Feb 09, 2024
Dupixent Breaks Ground: First and Only Eosinophilic Esophagitis Treatment for Pediatric Patients
Sanofi and Regeneron are prioritizing pediatric care, particularly in their recent progress with the potent anti-inflammatory drug, Dupixent. On January 25, 2024, the FDA approved Dupixent, an IL-4 receptor alpha antagonist, for the treatment of eosinophilic esophagitis (EoE) in children aged 1 to 11, weighing at l...
Read More...
Feb 08, 2024
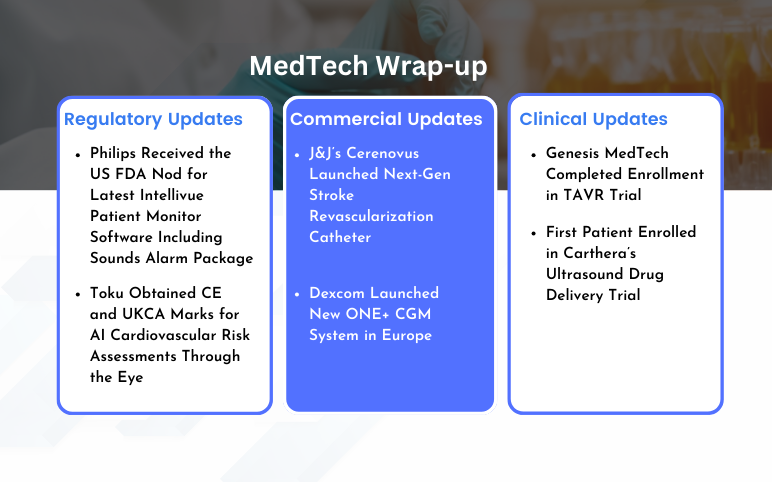
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...
Feb 07, 2024
Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
As we step into the crisp corridors of 2024, the healthcare landscape unfolds a compelling saga of mergers, strategic funding, and transformative acquisitions. In this month-by-month analysis, we delve into the intricate tapestry of industry dynamics, exploring the impactful maneuvers that are shaping the healthcar...
Read More...
Feb 06, 2024
4DMT Presents Data From Phase II PRISM Clinical Trial; Adaptimmune Announces FDA Acceptance of Biologics License Application for Afami-cel; Astellas Submits Supplemental New Drug Application in Japan for PADCEV with KEYTRUDA; AnaMar Announces US and EU Orphan Drug Designation for AM1476; Biosyngen Announces FDA Fast Track Designation for BST02; Acepodia Announces FDA Clearance of IND Application for ACE2016
4DMT Presents Positive Interim Data from Randomized Phase II PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD 4D Molecular Therapeutics, a prominent company in the field of genetic medicines with a focus on harnessing the full potential of geneti...
Read More...
Feb 05, 2024
Opportunities and Challenges for Cell and Gene Therapies
Gene therapy is an innovative medical approach that manipulates an individual’s genetic material to prevent or treat diseases. It primarily involves introducing, modifying, or substituting genes within a patient’s cells. The central objective of gene therapy is to rectify genetic abnormalities, insert missing genes...
Read More...
Feb 02, 2024
Unleashing the Power of mRNA: A Revolutionary Approach to Vaccines and Therapeutics
The progress of mRNA-based therapeutics involves several key stages, including mRNA design, synthesis, entrapment, pharmacodynamics, pharmacokinetics, in vivo and in vitro safety evaluation, manufacturing, and clinical trials. Among these stages, mRNA design and synthesis play pivotal roles in developing mRNA-based...
Read More...
Feb 01, 2024
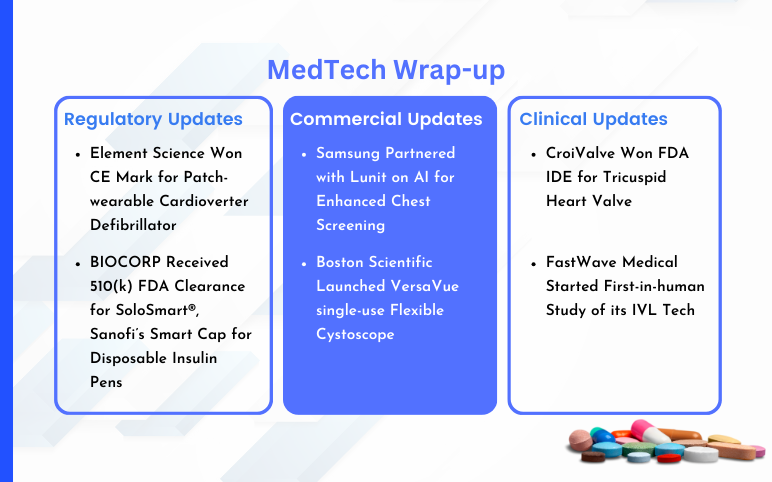
Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope; Element Science’s Patch-wearable Cardioverter Defibrillator; BIOCORP Received 510(k) FDA Clearance for SoloSmart; FDA IDE for CroiValve’s Tricuspid Heart Valve; FastWave Medical’s First-in-human Study of its IVL Tech
Samsung partnered with Lunit on AI for Enhanced Chest Screening On January 25, 2024, Samsung entered into a supply collaboration with Lunit, in order to employ its AI-powered technology for conducting chest screenings. The agreement was signed by Boston Imaging, which serves as the U.S. hub for Samsung's d...
Read More...