Meeting the Unmet Need for Oral Mucositis
Oct 29, 2018
Oral Mucositis (OM) refers to erythematous and ulcerative lesions of the oral mucosa observed in cancer patients under chemotherapy or radiation therapy. In its severe form, OM presents as confluent, deep ulcerations. Typical manifestations are atrophy, erythema, ulceration, and swelling of the mucosa. It appears firstly by thinning of oral tissues which lead to erythema. As these tissues become thinner, ulceration eventually occurs. The potential complications include pain, increased risk of local and systemic infections, bleeding; and insufficient food intake and may lead to breaks in treatment sessions.
DelveInsight estimates that approximately 1.9 Million patients in the United States were diagnosed with Oral Mucositis in 2016. Since the prevalence of cancer patients is on the rising mode across the globe, the market of OM therapeutics is also expanding proportionally. In addition, the pain derived from OM and the subsequent need to cure the condition at the earliest is the foremost driver of the growth of this market.
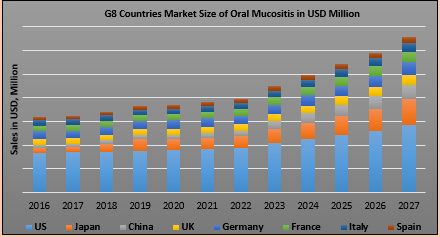
On the basis of geography, the global therapeutic market is segmented into eight major markets including, the United States, EU5 (Germany, France, Italy, Spain, and United Kingdom) Japan and China. Out of these, United States has the largest market on OM therapeutics, closely followed by the EU5 region.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Inspira™ Approval of INSPIRA™ ART100 System; Oticon Medical’s Sentio™ System Received Regul...
- Novartis Announces the Positive Results of Phase III NATALEE Trial Evaluating Kisqali; FDA Approv...
- Treatment for esophageal cancer; FDA bans soaps; Dr Reddy’s Launches; Cipla Gets Approval
- Cambrex acquires Avista; Sobi pays $50M; LEO Pharma signs deal; Boston Scientific remunerates
- Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; ...
- Oral Mucositis
There is no cure or preventive method approved for OM and the current treatment is palliative in nature. The current OM therapeutic market includes several kinds of drug formulations and treatments, such as cryotherapy, anti-inflammatory agents, mouthwashes and coating agents.
These symptomatic treatment options available in the market have not proven effective to control the incidence and trauma of the disease that appears with each cycle of cancer treatment. To counter such unmet need of the therapy, several companies are working diligently especially on the Head and Neck Cancer segment to ease the life of the cancer patients. Additionally, given the impact that oral toxicities have on daily functioning, there is a need for extensive research to focus not only on the incidence of oral mucositis and stomatitis but also on the severity and duration of the disease.
The major key players in the global OM therapeutic market are Access Pharmaceuticals, Soligenix Inc., Swedish Orphan Biovitrium Ltd., Oragenics, Monar Therapeutics, and Enzychem Lifesciences.
Currently, the market preparations for Oral Mucositis are Dusquetide, Mosedipimod, SAMITAL, GC4419, AG013, RRx-001, and Rebamipide. The United States Food and Drug Administration has granted “Fast Track” Designation for SGX942 (Soligenix, Inc.) for the treatment of oral mucositis in patients with head and neck cancer. This drug along with various mouth rinse and pipeline candidates are expected to further drive the OM therapeutics market. The market size of Oral Mucositis (OM) in the eight major markets was approximately USD 630 million in 2016.
Downloads
Article in PDF
Recent Articles
- Boston Scientific Corporation Agreement to Acquire Silk Road Medical; Philips Introduced Duo Veno...
- Snippet
- FDA grants approval; Sanaria receives fast-track designation; Sanofi and Regeneron’s next blockbu...
- BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra;...
- Wearing Technology to stay fit