Small Cell Lung Cancer Treatment Market Propels Due to Extremely Robust Pipeline
Mar 14, 2022
Table of Contents
Small Cell Lung Cancer (SCLC) is a particularly aggressive type of lung cancer. It is distinguished by the rapid and uncontrolled growth of certain cells in the lungs. A tumor forms eventually, and cancer can spread to other parts of the body. Small Cell Lung Cancer treatment usually involves chemotherapy, radiotherapy, immunotherapy and surgery. Tobacco use is the primary Small Cell Lung Cancer risk factor; almost all affected individuals smoke or have a history of smoking. Small Cell Lung Cancer symptoms vary from person to person, and there are rarely any symptoms early in the disease’s progression. The common SCLC symptoms include a persistent cough, chest pain that worsens when coughing, laughing or taking a deep breath, shortness of breath, blood in the cough (hemoptysis), and hoarseness or wheezing. Some people who are affected may experience appetite loss, unintended weight loss, fatigue, and recurrent episodes of lung infections such as pneumonia or bronchitis. There are two Small Cell Lung Cancer stages: limited-stage disease, which is potentially curable in about 20% to 25% of people, and extensive-stage disease, which is more difficult to treat. SCLC can be fatal in a few weeks if untreated, in contrast to most NSCLC cases.
Current Small-Cell Lung Cancer Market
The Small Cell Lung Cancer market is anticipated to rise in the coming years owing to various factors such as increasing incident cases of SCLC which is observed mainly due to an increase in the geriatric population, expected entry of potential premium price emerging therapies, and readily uptake of currently approved immunotherapies worldwide.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Small Cell Lung Cancer Market Booms with an Influx of Companies and Robust Pipeline
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- 7 Companies at the Forefront of Trispecific Antibody Development
- Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s...
- Amgen’s IMDELLTRA FDA Approval; J&J’s Proteologix Acquisition; Bristol Myers Squibb’s B...
According to DelveInsight’s Small Cell Lung Cancer epidemiological analysis, there is a gender difference observed in the disease. It was seen that SCLC occurs more frequently in the male population as compared to females. Also, It has been observed that about three-quarters of Small Cell Lung Cancer patients are diagnosed with extensive-stage SCLC. In addition to this, most incident cases of SCLC were recorded in the United States among the 7MM countries.
Small Cell Lung Cancer has been observed across all ages and this makes age, one of the most prominent factors for this condition. During the understanding of this indication and analyses of the various research paper, it was found that SCLC cases are most common in middle-aged and older adults. In comparison, they are relatively less common in young adults
Small-Cell Lung Cancer Marketed Therapies
At present, there are a number of Small-Cell Lung Cancer treatment options available in the market. These are classified on the basis of a patient’s specificity of lung cancer such as the stage of cancer, the patient’s overall health, including how well the organs of the patient’s body are functioning, and the patient’s preferences. Current SCLC treatment options include surgery, chemotherapy, radiation therapy, and immunotherapy as the widely accepted therapeutic treatment choices. This can also vary on the basis of symptoms, age, stage, how fast it is growing, and whether patients may suffer various patterns of recurrence requiring subsequent lines of rescue therapies. The most commonly used chemotherapy regimen is Etoposide or Irinotecan plus a platinum-based drug such as Cisplatin or Carboplatin. For people with limited-stage SCLC, chemotherapy plus radiation therapy to the chest is given daily over several weeks. People with extensive-stage cancer initially receive chemotherapy for 3 to 4 months or they may receive chemotherapy in combination with immunotherapy.
In 2017, the FDA approved the drug Nivolumab (Opdivo) by Bristol Myers Squibb for the treatment of individuals with Small Cell Lung Cancer in whom cancer has spread (metastasized) and who have been previously treated with two types of therapy including chemotherapy.
On June 17, 2019, the FDA granted accelerated approval to Keytruda to pharma giant Merck for patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy and at least one other prior line of therapy.
In March 2020, AstraZeneca’s Imfinzi has been FDA approved in the United States as the first-line treatment for adult patients with Extensive-Stage Small Cell Lung Cancer (ES-SCLC) in combination with standard-of-care (SoC) chemotherapies, etoposide plus either carboplatin or cisplatin (platinum-etoposide).
Potential Small-Cell Lung Cancer Emerging Therapies
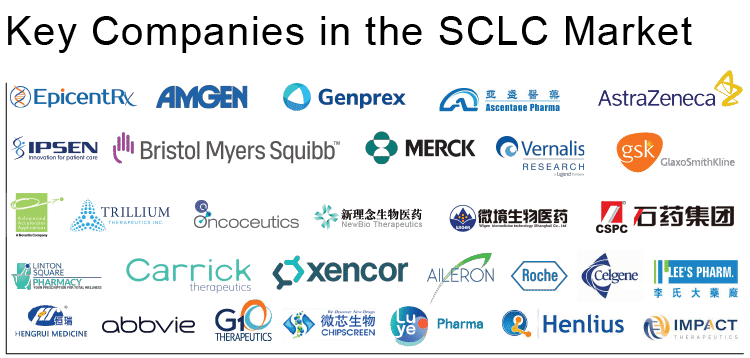
The dynamics of the SCLC market are anticipated to change in the coming years owing to the improvement in the rise in the number of healthcare spending across the world and the expected entry of novel and potential SCLC pipeline therapies. The leading companies, such as Ipsen Biopharmaceuticals, Bristol-Myers Squibb, EpicentRx, Amgen, Genprex, and others are developing novel pipeline drugs for Small Cell Lung Cancer treatment. Some of the therapeutic SCLC pipeline drugs include-
RRx 001: EpicentRx
EpicentRx is developing RRx-001, a next-generation small-molecule immunotherapy. The therapy works by repolarizing tumor-associated macrophages (TAMs) and other immunosuppressive cells in the tumor microenvironment to an immunostimulatory phenotype. RRx-001 stimulates the immune system and can be used alone or in combination with chemotherapy, immunotherapy, radiation, and targeted agents, giving the therapy the potential to transform “treatment-resistant” tumors into “treatment-sensitive” tumors.
RRx-001 is currently being studied in the REPLATINUM Phase III trial for the treatment of third-line and advanced Small Cell Lung Cancer (SCLC). RRx-001 is also being studied in Phase II QUADRUPLE THREAT trial for non-small cell lung cancer, neuroendocrine tumors, ovarian, prostate cancer, and Small Cell Lung Cancer treatment.
REPLATINUM is comparing the efficacy and safety of RRx-001 plus platinum doublet chemotherapy to platinum doublet chemotherapy alone in an open-label, multicenter, crossover trial designed in collaboration with the FDA. The primary endpoint of REPLATINUM is progression-free survival, with overall survival and overall response rate serving as secondary endpoints. It includes 126 participants aged between 18 to 79 years and the study is estimated to be completed by March, 2024.
The REPLATINUM trial comes on the heels of EpicentRx’s ongoing Phase 2 QUADRUPLE THREAT trial, which is testing RRx-001 plus platinum chemotherapy in a variety of cancer types, including SCLC. It includes 213 participants of age 18 years and older and the Phase 2 QUADRUPLE THREAT trial will be complete by June 2022.
Onivyde: Ipsen
The French pharmaceutical Ipsen is currently investigating a drug candidate – Onivyde for Small Cell Lung Cancer treatment. USFDA granted a Fast Track Designation (FTD) in December 2020 to Onivyde, as a second-line monotherapy alternative for patients with SCLC whose disease progressed following a platinum-based chemotherapy regimen. This trial is currently being investigated in Phase II/III RESILIENT trial.
This clinical trial is a randomized, open-label study of Irinotecan liposome injection (Onivyde) versus intravenous topotecan in patients with Small Cell Lung Cancer who have progressed on or after platinum-based first-line therapy with 480 participants of age 18 years and older. The expected completion of this trial is estimated to be done by December 2022.
BMS-986012: Bristol-Myers Squibb
Bristol-Myers Squibb is considered to be a pioneer if lung-cancer therapeutic drugs are involved. Aside from the major production of Opdivo, BMS is currently investigating another drug candidate – BMS-986012 in combination with carboplatin, etoposide, and nivolumab as first-line therapy in Extensive-stage SCLC. BMS-986012 is a first-in-class, fully human monoclonal antibody with enhanced antibody-dependent cell-mediated cytotoxicity that binds to fucosyl-GM1, a ganglioside highly expressed on Small Cell Lung Cancer.
BMS-986012 is currently being investigated in a Phase I/II multicenter study of BMS-986012 in subjects with relapsed/refractory Small Cell Lung Cancer. The purpose of this study is to determine the safety, tolerability, pharmacokinetics, immunogenicity, antitumor activity and pharmacodynamics of BMS-986012 alone and in combination with nivolumab in patients with relapsed/refractory SCLC. It includes 107 participants aged 18 years and older, the study is estimated to be completed by June, 2022.
Another clinical trial study for BMS-986012 is a Phase II, randomized, open-label, in combination with carboplatin, etoposide, and vivolumab as first-line therapy in Extensive-stage Small Cell Lung Cancer treatment. This study includes 120 participants aged 18 years and older, the study is estimated to be completed by March, 2025.
AMG 757: Amgen
AMG 757 is currently being tested for SCLC by Amgen. AMG 757 is a Bispecific T cell engager (BiTE) with a Half Life Extended (HLE) targeting delta-like protein 3 (DLL3). It is being studied in an open-label, ascending, multiple-dose Phase I trial in combination with anti-PD1 treatment. AMG 757 will be given as a short-term treatment. A non-randomized, interventional study, it includes 50 participants that are of 18 years and older age. This study is anticipated to be completed by October, 2025.
REQORSA: Genprex
Genprex recently announced that it has expanded its oncology research and development pipeline to include Small Cell Lung Cancer (SCLC) as an additional disease indication for REQORSA Immunogene Therapy, it’s lead drug candidate. SCLC accounts for about 10-15% of the lung cancer market, whereas REQORSA’s initial target indication of Non-Small Cell Lung Cancer (NSCLC) accounts for about 84% of the lung cancer market.
REQORSA consists of a plasmid encapsulated in non-viral lipid nanoparticles that expresses the TUSC2 gene. Genprex’s oncology program makes use of its proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, which the company believes is the first systemic gene therapy delivery platform used in humans for cancer. The ONCOPREX Nanoparticle Delivery System could enable the delivery of a number of cancer-fighting genes, either alone or in combination with other cancer therapies, to potentially address unmet medical needs in cancer, potentially improving patient outcomes through the advancement of multiple therapeutic approaches for large patient populations.
REQORSA has the potential to treat both Small Cell Lung Cancer and Non-small Cell Lung Cancer, making it a potential drug candidate for the entire lung cancer market.
There is an exceptionally robust pipeline of Small Cell Lung Cancer treatment therapies. Some of the other pipeline therapeutic drugs which are in the Phase III of clinical trials include Chiauranib Capsule currently being investigated by Chipscreen Biosciences, Ltd., Durvalumab and Tremelimumab evaluated by AstraZeneca, Shanghai Henlius Biotech’s investigational drug HLX10 (Recombinant Humanized Anti-PD-1 Monoclonal Antibody Injection) in combination with chemotherapy (Carboplatin-Etoposide) in previously untreated patients with Extensive Stage Small Cell Lung Cancer (ES-SCLC), and a number of other therapies.

What’s Ahead?
Although there have been significant advances in the treatment of metastatic NSCLC over the last decade, they have not been as successfully translated to Small Cell Lung Cancer treatment. However, both the European Lung Cancer Virtual Congress 2021 (ELCC 2021) and the American Society of Clinical Oncology Annual Meeting 2021 (2021 ASCO) hinted that SCLC treatment may change in the next 3 to 5 years.
Moreover, ELCC 2021 featured exciting news about ongoing advances in SCLC. The development of epigenetic erasers, enzymes capable of removing epigenetic marks by reversing their influence on gene expression is being used in the potential Small Cell Lung Cancer treatment in the field of epigenetics.
Furthermore, treatment advances are being made on multiple fronts in the fight against SCLC. Adoptive immunotherapy, tumour subtyping, combinatorial immunotherapies, emerging risk stratification cues, and new checkpoint inhibitor medications are all being researched. There is still hope for Small Cell Lung Cancer treatment. The disease is in the early stages of discovery and clinical trials, and human effort will propel development forward. In addition, clinical trials will pave the way to a more effective treatment strategy for Small Cell Lung Cancer treatment.
FAQs
Small Cell Lung Cancer (SCLC) is a particularly aggressive type of lung cancer. It is distinguished by the rapid and uncontrolled growth of certain cells in the lungs. A tumor forms eventually, and cancer can spread to other parts of the body.
The Small Cell Lung Cancer symptoms vary from person to person, and there are rarely any symptoms early in the disease’s progression. The common SCLC symptoms include a persistent cough, chest pain that worsens when coughing, laughing or taking a deep breath, shortness of breath, blood in the cough (hemoptysis), and hoarseness or wheezing.
Some of the novel therapeutic drugs in the SCLC pipeline include names like RRx 001, Onivyde, BMS-986012, AMG 757, REQORSA, and others.
The leading companies such as Merck, Ipsen Biopharmaceuticals, Bristol-Myers Squibb, EpicentRx, Amgen, Genprex, and others are developing drugs for Small Cell Lung Cancer treatment.
Downloads
Article in PDF
Recent Articles
- Copiktra receives approval; Lilly wins approval; Eisai’s Fycompa; Astellas’ Roxadustat; Janssen’s...
- Amgen’s IMDELLTRA FDA Approval; J&J’s Proteologix Acquisition; Bristol Myers Squibb’s B...
- Small Cell Lung Cancer Market Booms with an Influx of Companies and Robust Pipeline
- Top 5 Cancers Creating Major Challenge To The Global Healthcare System
- AstraZeneca’s IMFINZI Archives Another Milestone — Becomes the First Immunotherapy for Limited-St...