3D Cardiac Mapping Systems
Oct 24, 2024
iRhythm’s Zio® AT Device Improvements Receive FDA Clearance; Epitel Adds REMI AI Device to Portfolio with Fourth FDA 510(k); SeaStar Medical Advances Acute Kidney Injury Trial, Approaches Interim Analysis; Endospan Completes Primary Enrollment for TRIOMPHE Study of NEXUS® Stent Graft; Boston Scientific Enhances FARAPULSE™ PFA with New Cardiac Mapping Technology; GE HealthCare Debuts CareIntellect to Support Oncologists with AI-Enhanced Patient Insights
iRhythm Technologies Received FDA 510(k) Clearance for Design Updates Previously Made to Its Zio® AT Device On October 21, 2024, iRhythm Technologies, Inc., a leading digital healthcare company specializing in solutions to detect, predict, and prevent disease, announced that the U.S. Food and Drug Administ...
Read More...
Jan 13, 2022
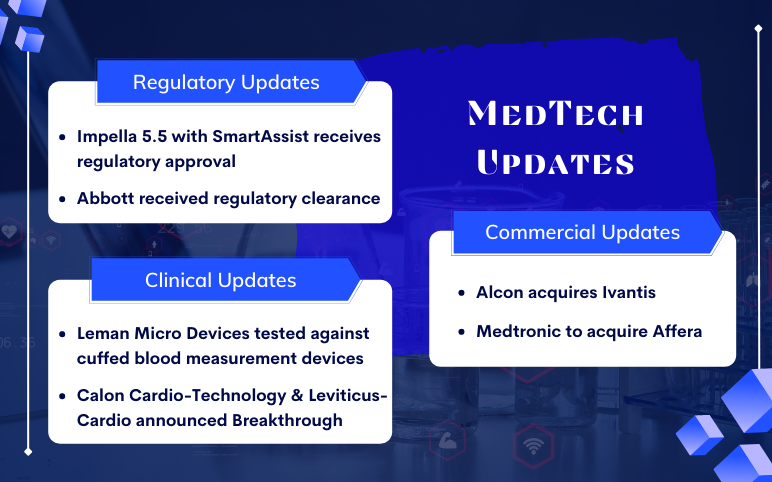
Leman Micro Devices’ e-Checkup system; Calon Cardio-Technology & Leviticus-Cardio announced Breakthrough; Alcon buys Ivantis; Medtronic to acquire Affera; Abiomed’s Impella 5.5 with SmartAssist; Abbott’s EnSite X EP System with EnSite OT
Leman Micro Devices tested against the cuffed blood measurement devices reported positive results On January 07, 2022, the e-Checkup system by Leman Micro Devices tested in the world’s first clinical trial against the cuffed blood measurement devices reported positive results. The e-Checkup and its sma...
Read More...

-Agonist.png)

