AstraZeneca
Oct 17, 2023
Novo Nordisk to Acquire Ocedurenone; FDA Awards Orphan Drug Designation to SLS009 in AML; FDA Approves Adjuvant Nivolumab in Completely Resected Stage IIB/C Melanoma; Fast Track Designation to South Rampart Pharma’s SRP-001; TAGRISSO + Chemotherapy Granted Priority Review in the US; Fast Track Designation to SurVaxM for Glioblastoma
South Rampart Pharma Receives U.S. FDA Fast Track Designation for SRP-001 for Acute Pain On October 12, 2023, South Rampart Pharma, Inc. announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track status to its drug candidate, SRP-001, intended for the management of acute pain. SRP-001 is a...
Read More...
Jul 21, 2023
Beyfortus: A New Respiratory Syncytial Virus (RSV) Drug for Toddlers
Sanofi’s immunization strategy is taking shape, owing to an authorization that could drive the company and its partner AstraZeneca to the forefront of the respiratory syncytial virus (RSV) treatment battle. The FDA approved Sanofi and AstraZeneca’s monoclonal antibody Beyfortus, also known as nirsevimab, as a preve...
Read More...
Jul 18, 2023
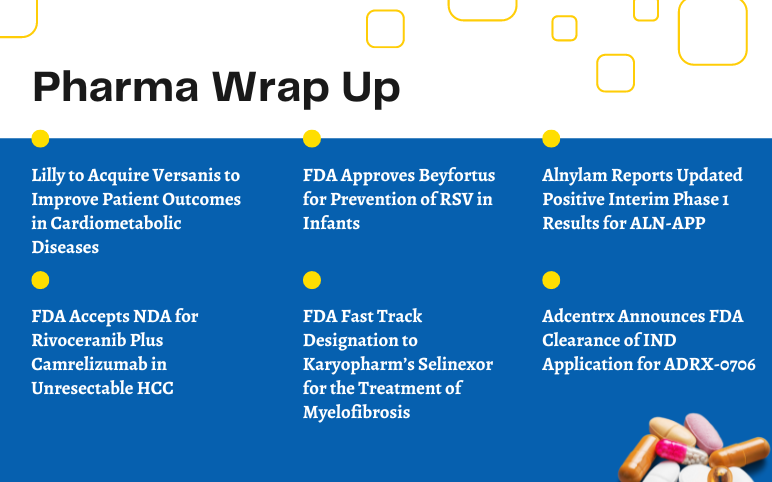
Lilly to Acquire Versanis; FDA Approves Beyfortus for RSV in Infants; Alnylam Presented Updates on Interim Phase 1 Results for ALN-APP; FDA Accepts NDA for Rivoceranib Plus Camrelizumab; FDA Fast Track Designation to Karyopharm’s Selinexor; Adcentrx’s IND Application for ADRX-0706
Lilly to Acquire Versanis to Improve Patient Outcomes in Cardiometabolic Diseases Eli Lilly & Company and Versanis Bio announced a definitive agreement for Lilly to acquire Versanis, a private clinical-stage biopharmaceutical firm focused on the discovery of novel medications for the treatment of cardiometab...
Read More...
Jan 31, 2023
Eli Lilly’s Jaypirca Approval; Novartis’ Adakveo EMA Review; Janssen’s CARTITUDE-4 Study of CARVYKTI; Negative Review on Ipsen’s Palovarotene; Gilead Sciences and Kite’s Yescarta NICE Recommendations; Daiichi Sankyo and AstraZeneca’s Enhertu EU Approval
FDA Approves Eli Lilly’s Jaypirca for Relapsed Mantle Cell Lymphoma Eli Lilly has received FDA approval for Jaypirca, a non-covalent BTK inhibitor, in relapsed mantle cell lymphoma (MCL) patients who have relapsed after treatment with other drugs in the class. Adult MCL patients who have previously received at l...
Read More...
Jan 17, 2023
FDA Approves Luye’s Rykindo; EU Approves AstraZeneca’s Tezspire; Oramed Announces Trial Results of ORMD-0801; Eisai and Biogen File Lecanemab in the EU; AbbVie and Anima Biotech Announce Collaboration; AstraZeneca’s Avillion Receives FDA Approval
FDA Approves Luye Pharma’s Rykindo for Schizophrenia Luye Pharma has received its first FDA approval for Rykindo, an injectable formulation of the antipsychotic risperidone administered every two weeks. Rykindo has been approved by the US Food and Drug Administration for the treatment of schizophrenia as well as...
Read More...
Dec 27, 2022
Gilead Sciences’ Sunlenca Approval; FDA Approves Roche’s CD20xCD3 Bispecific Antibody Lunsumio; EU Approves AstraZeneca’s Imfinzi Plus Chemo; Pfizer Files Blockbuster Hope Etrasimod for Ulcerative Colitis; FDA Approves Mosunetuzumab for R/F Follicular Lymphoma; FDA Breakthrough Therapy Designation to Adagrasib Plus Cetuximab for KRAS G12C–Mutated Advanced CRC
FDA Approves Gilead Sciences’ Sunlenca Sunlenca, a Gilead Sciences therapy for people with multidrug-resistant (MDR) HIV infection that only needs to be taken twice a year, has received FDA approval for the second time of asking. Sunlenca, which is based on the HIV capsid inhibitor lenacapavir, is intended to be...
Read More...
Mar 23, 2021
Evotec, Takeda Deal; AZ COVID Vaccine US Trials; Promega’s Recognition; Roche’s Tecentriq Results in NSCLC
Promega Biotech Ibérica Gains Recognition for COVID-19 Response in Spain Promega Biotech Ibérica has been heralded as the business leader in Spain for its COVID-19 response. The company’s innovative approach to deal with COVID-19 and pandemic has led La Razón, a daily newspaper based in Madrid, to felicitate the...
Read More...
Mar 02, 2021
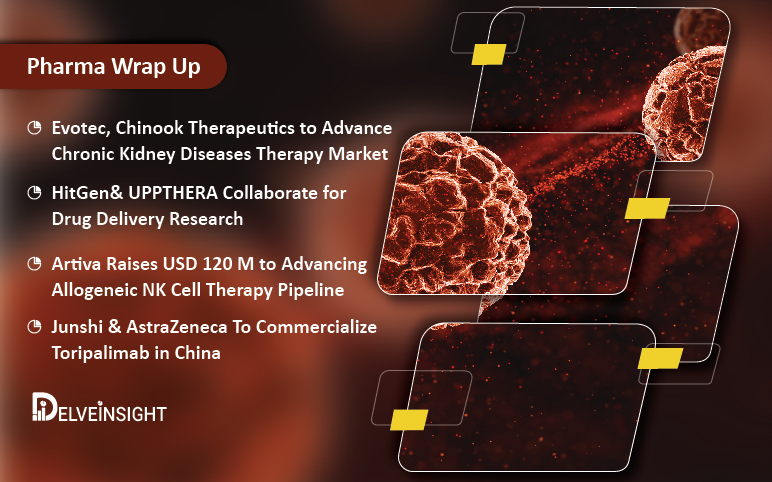
Evotec, Chinook Teams Up for CKD Therapy; HitGen & UPPTHERA in Drug Delivery Research; Artiva’s $120 M Coup of NK Cell Therapy; Junshi & AZ with Toripalimab in China
Evotec, Chinook Therapeutics to Advance Chronic Kidney Diseases Therapy Market Evotec and Chinook Therapeutics have collaborated to develop and discover precision medicine therapies for patients with chronic kidney diseases (CKD). The duo plans to jointly identify, characterize and validate novel mechanisms and ...
Read More...
Jan 19, 2021
Biohaven’s Troriluzole Failure; Daiichi/ AZ’s Enhertu; Fujifilm & Manufacturing Spree; J&J’s Darzalex Faspro
Biohaven's Troriluzole Dwindles Again In Alzheimer’s After Anxiety Biohaven Pharmaceuticals had put too much faith in its third-generation prodrug, Troriluzole. The company has tested the efficacy of the drug in more than one indication, including generalized anxiety disorder (GAD), obsessive-compulsive di...
Read More...
Dec 15, 2020
FTA for Gannex’s ASC4; Disappointment for Incyte’s Ruxolitinib; Historic win for Pfizer, BioNTech’s COVID Vaccine; Alexion buyout; the debut of InnoSkel
Gannex received US FDA fast track designation for its NASH drug, ASC42 an FXR Agonist Gannex Pharma has received Fast Track designation approval from FDA for its drug candidate ASC42 for non-alcoholic steatohepatitis (NASH). The FTA designation will help the pharma company to advance its research and development...
Read More...







-Agonist.png)

