Biopsy Devices Market
Nov 12, 2025
Biopsy Devices: Advancing Diagnostic Precision in Modern Healthcare
The field of oncology and pathology hinges on one definitive step: the biopsy. Serving as the gold standard for diagnosing a vast majority of cancers and other diseases, the process of obtaining tissue samples has driven continuous technological innovation. The biopsy device market represents a vital sector within ...
Read More...
Aug 08, 2024
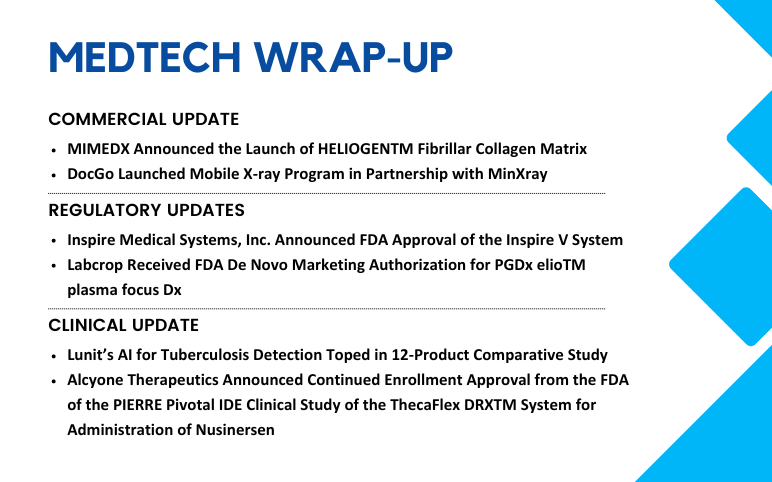
MIMEDX Launches HELIOGENTM Fibrillar Collagen Matrix; DocGo Launches Mobile X-ray Program; Labcrop’s FDA De Novo Marketing Authorization; Inspire Medical Systems Receives FDA Approval; Alcyone Therapeutics Announces Continued Enrollment Approval from the FDA; Lunit’s AI for Tuberculosis Detection
MIMEDX Announced the Launch of HELIOGENTM Fibrillar Collagen Matrix On July 31, 2024, MiMedx Group, Inc. launched HELIOGEN™ Fibrillar Collagen Matrix, a particulate xenograft product designed to treat complex wounds, especially in surgical environments. HELIOGEN is a shelf-stable product that contains Type...
Read More...
Oct 19, 2023
Mindray Collaborated with Edwards Lifesciences; DuPont’s Low-Cyclics Silicone Soft Skin Adhesive; GE HealthCare’s Allia IGS Pulse; Praxis Medical’s EndoCore EBUS-TBNA Biopsy Device; Visioneering’s PROTECT Clinical Trial; SynerFuse’s e-TLIF procedure
DuPont Launched Higher-Adhesion and Low-Cyclics Silicone Soft Skin Adhesive On October 17, 2023, DuPont, a globally recognized leader in technology for a broad range of innovations in medical devices, biopharmaceutical processing, and pharmaceutical solutions launched Liveo™ MG 7-9960 Soft Skin Adhesive. ...
Read More...
Sep 14, 2023
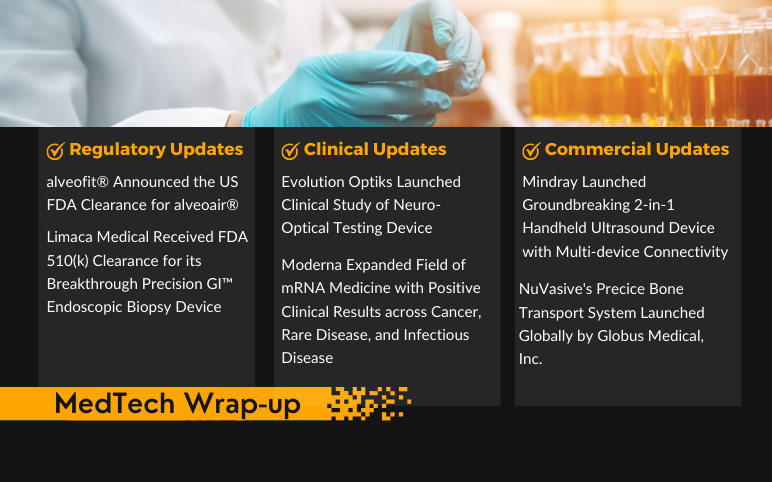
Mindray’s 2-in-1 Handheld Ultrasound Device; NuVasive’s Precice Bone Transport System; FDA for alveofit’s alveoair; FDA 510(k) Clearance Limaca’s Precision GI™ Endoscopic Biopsy Device; Evolution Optiks’s Neuro-Optical Testing Device; Moderna Expanded Field of mRNA Medicine
Mindray Launched Groundbreaking 2-in-1 Handheld Ultrasound Device with Multi-device Connectivity On September 7, 2023, Mindray, a global leader and developer of healthcare solutions and technologies in ultrasound, patient monitoring, and anesthesia announced the release of its new imaging solution, the TE ...
Read More...
Oct 27, 2022
FDA Approval to DePuy TELIGEN System; FDA Breakthrough Device Designation for the EndoStim System; GE and Accuray Announces Collaboration; Mentice and Acandis Announces a Three-Year Agreement; Indigo Diabetes Enrolled First Participant in the Shine Clinical Trial; FDA IDE Approval to MedAlliance’s SELUTION SLR
DePuy Synthes Received FDA Approval for TELIGEN™ System On October 20, 2022, DePuy Synthes, The Orthopaedics Company of Johnson & Johnson, provides one of the most comprehensive orthopedics portfolios in the world that helps heal and restore movement for the millions of patients, announced that ...
Read More...



-Agonist.png)

