Brain Monitoring Device
Jul 07, 2022
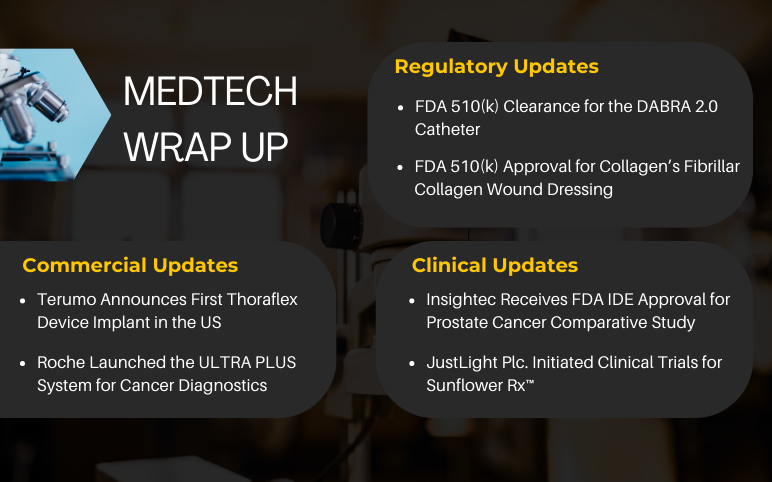
Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnostics ULTRA PLUS system launch; Insightec IDE Approval for Prostate Cancer; JustLight plc. trial of Sunflower Rx for Alzheimer’s disease; Thoraflex Hybrid Device Implantation in the United States; FDA 510(k) Clearance for the DABRA 2.0 Catheter
Collagen Matrix received FDA 510(k) approval for Fibrillar Collagen Wound Dressing On June 29, 2022, Collagen Matrix, Inc., a leader in regenerative medicine, a global manufacturer of collagen and other biomaterial-based medical devices, and Linden Capital Partners portfolio company announced FDA 510(k) Clearan...
Read More...
-Agonist.png)

