Cancer Diagnostic Market
Sep 10, 2025
From Lab to Bedside: The Rise of AI-Enabled Digital Pathology
Pathology—the discipline of diagnosing disease through tissue analysis, has long depended on glass slides and microscopes. Today, digital pathology is undergoing a remarkable shift, as whole-slide imaging (WSI) and artificial intelligence (AI) come together to streamline lab workflows, accelerate diagnoses, and enh...
Read More...
May 03, 2023
Cancer Diagnostic Market: Evaluating the Major Growth Factors and the Key Developments in the Domain
Cancer is a global burden, leading to a significant number of death and disabilities worldwide. In 2020, nearly one in six deaths were related to cancer. Breast cancer, lung cancer, colon and rectum cancer, and prostate cancer are some of the major cancer types, registering the highest number of cases globally. To ...
Read More...
Dec 01, 2022
AnchorDx’s UriFind Bladder Cancer Assay in the US; UroMems Initiates Smart Implant to Treat Stress Urinary Incontinence; FDA 510(k) Clearance to NeuroLogica’s BodyTom 64; CE-IVD Mark Approval to SeekInCure’s Recurrence Monitoring Kit Gets; Ypsomed and CamDiab’s Automated Insulin Dosing System; Boston Scientific to Acquire Apollo Endosurgery
AnchorDx Clinical Trial Enrols First Patient for its UriFind® Bladder Cancer Assay in the US On November 23, 2022, AnchorDx, announced the first patient enrollment for its clinical trials of the UriFind® bladder cancer assay in the United States. The UriFind® bladder cancer assay clinical trial in the U...
Read More...
Aug 25, 2022
Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to Acquire enCAGE Coil Precision Ablation System; Boston Heart Diagnostics Launches LipidSeqTM; Rocket VR Health Partners with Penn Medicine’s Abramson Cancer Center; Thermedical’s Clinical Trial to Treat Ventricular Tachycardia
Edwards Pascal Precision Transcatheter Mitral And Tricuspid Valve Repair System Receives CE Mark On August 17, 2022, Edwards Lifesciences Corporation, an American medical technology company headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company announc...
Read More...
Jul 21, 2022
FDA Approval to Centinel’s Total Disc Replacement Devices; FDA clears DyAnsys’ Neurostimulator; Orthox to Commence FFLEX Study; Cordis Started Enrolment for RADIANCY Clinical Study; Orthofix & LimaCorporate Announce Partnership; Medtronic to Partner With AWS
Orthox Obtains MHRA Approval to Commence FFLEX Study On July 14, 2022, Orthodox Ltd, a clinical-stage company created medical implants to treat orthopedic problems including damaged knee articular cartilage. It was founded in 2008 by life scientist, Dr. Nick Skaer, and knee surgeon, Professor Oliver Kessle...
Read More...
Jun 30, 2022
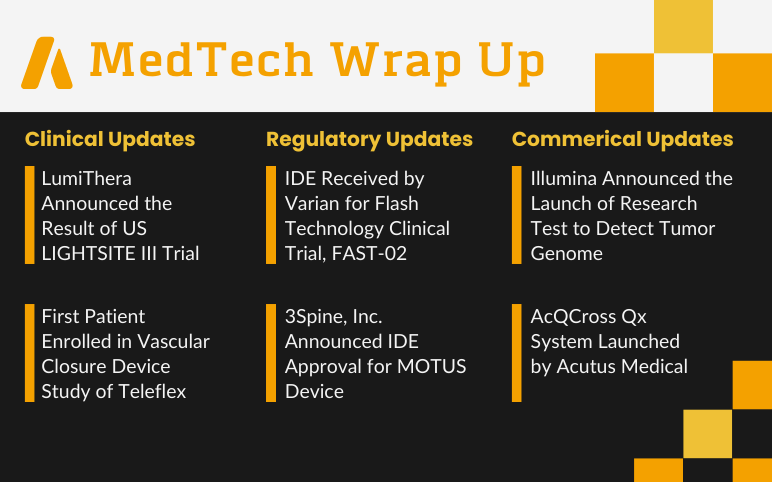
LumiThera’s US LIGHTSITE III Trial; First Patient Enrolled in Vascular Closure Device Study of Teleflex; Varian’s Flash Technology Clinical Trial, FAST-02; 3Spine’s MOTUS Device; Illumina’s Research Test to Detect Tumor Genome; Acutus Medical’s AcQCross Qx System
LumiThera Announced the Result of US LIGHTSITE III Trial of Non-neovascular Age-Related Macular Degeneration (AMD) Subjects Treated with Photobiomodulation (PBM) using the Valeda® Light Delivery System On June 22, 2022, LumiThera Inc., a commercial-stage medical device company offering photobiomodulation (PBM) t...
Read More...
May 19, 2022
Check-Cap’s Pivotal Trial for C-Scan; Curebase and Flow Neuroscience’s tDCS Device; Brainlab Acquires Majority Share in medPhoton; GE Healthcare to Invest in Pulsenmore; Medtronic’s Drug-Eluting Coronary Stent System; Remedee’ Endorphin Stimulation Solution;
Check-Cap Initiated the US Pivotal Trial for C-Scan® On May 11, 2022, Check-Cap Ltd., a clinical-stage diagnostic company initiated the US pivotal trial for C-Scan® at Mayo Clinic in Rochester Minnesota. C-Scan is the first and only patient-friendly, preparation-free screening test to detect polyps before they m...
Read More...





-Agonist.png)

