Cardiac Arrhythmia Monitoring Devices
Oct 09, 2025
Vektor Medical Receives CE Mark for vMap; Tosoh’s GR01 HbA1c Analyzer Gains FDA 510(k) Clearance; Zimmer Biomet Acquires Monogram Technologies; Olympus Partners with W. L. Gore & Associates to Distribute GORE® VIABIL® Biliary Stent Globally; Medtronic Launches U.S. IDE Study on Hugo™ Robotic Surgery System for Gynecology; Envoy Medical Secures FDA Clearance to Advance Pivotal Trial to Final Stage Following Promising Three-Month Results
Vektor Medical Secures CE Mark for vMap, Bringing the Benefits of Non-Invasive Arrhythmia Mapping to Europe On October 7, 2025, Vektor Medical, a leading innovator in cardiac arrhythmia care, received CE Mark approval for its vMap® System, a non-invasive, AI-powered technology that converts standard 12-lea...
Read More...
May 30, 2024
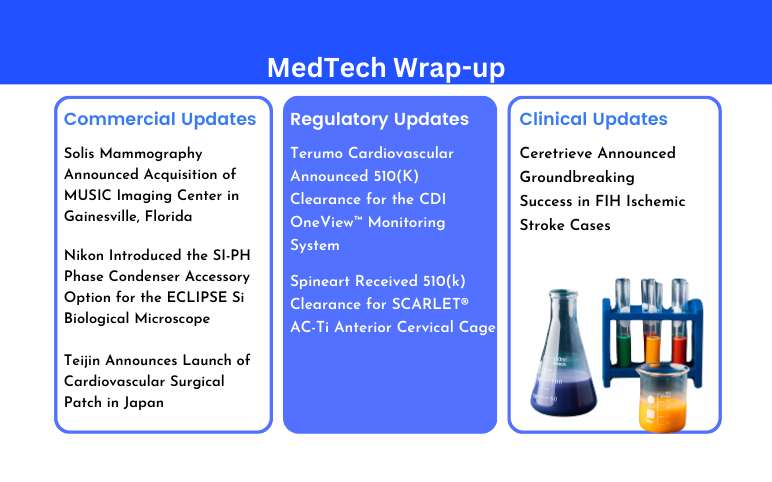
Solis Mammography’s Acquisition of MUSIC Imaging Center; Nikon’s SI-PH Phase Condenser Accessory Option; Teijin Launch Surgical Patch; Terumo Cardiovascular’s FDA 510(K) Clearance; Spineart’s 510(k) Clearance; Ceretrieve’s Success in FIH Ischemic Stroke Cases
Solis Mammography Announced Acquisition of MUSIC Imaging Center in Gainesville, Florida On May 28, 2024, Solis Mammography announced the acquisition of the MUSIC Mammography and Ultrasound Imaging Center in Gainesville, Florida. For more than 10 years, MUSIC has provided patients with an advanced standard ...
Read More...
May 23, 2024
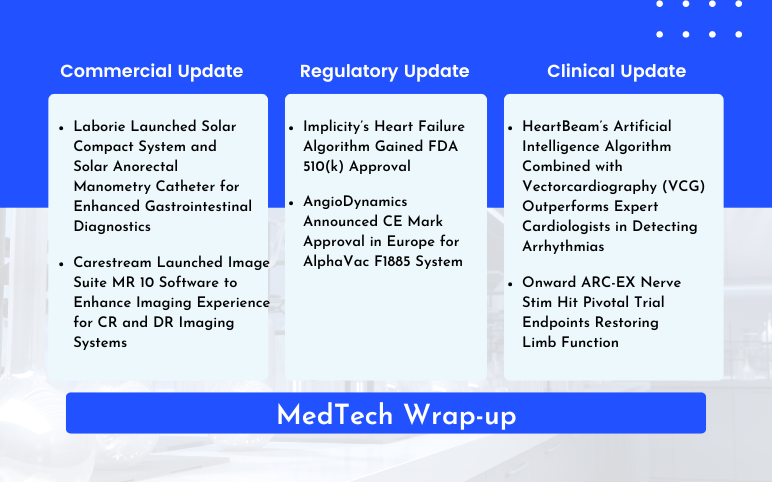
Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; Implicity’s Heart Failure Algorithm FDA 510(k) Approval; AngioDynamics’ AlphaVac F1885 System CE Mark Approval; HeartBeam’s Artificial Intelligence Algorithm for Arrhythmias; Onward ARC-EX Nerve Stim Hit Pivotal Trial
Laborie Launched Solar Compact System and Solar Anorectal Manometry Catheter for Enhanced Gastrointestinal Diagnostics On May 20, 2024, Laborie Medical Technologies Corp., launched the Solar Compact System and Solar Anorectal Manometry Catheter, the first disposable HRAM catheter on the market. These devices sig...
Read More...
Aug 31, 2023
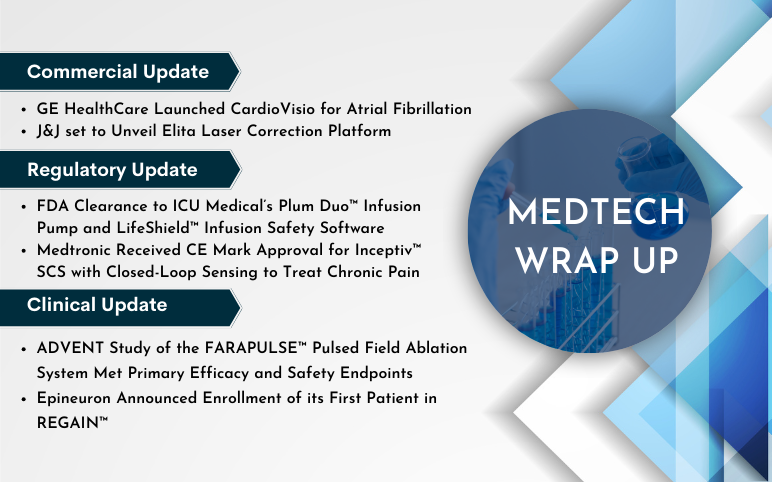
GE HealthCare Launched CardioVisio; J&J’s Elita Laser Correction Platform; ICU Medical’s Plum Duo Infusion Pump and LifeShield Infusion Safety Software Update; Medtronic’s Inceptiv SCS with Closed-Loop Sensing to Treat Chronic Pain; Boston Scientific’s FARAPULSE Pulsed Field Ablation System; Epineuron’s REGAIN Trial
GE HealthCare Launched CardioVisio for Atrial Fibrillation, a Digital, Patient-Centric Clinical Decision Support Tool to Enable Precision Care On August 24, 2023, GE HealthCare launched CardioVisio for Atrial Fibrillation (AFib), a digital tool designed to assist clinicians in visualizing longitudinal data relev...
Read More...
Sep 01, 2022
Miracor Medical’s Picso Pivotal Study; Medtronic’s Extravascular ICD; Galaxy Medical’s CENTAURI Pulsed Electric Field System; UltraSight’s Novel Cardiac AI Technology; Oticon Launched the World’s Smallest Hearing Aid; Medtronic Completes Acquisition of Affera
FDA Approved IDE for Picso® Pivotal Study of Miracor Medical On August 23, 2022, Miracor Medical SA, a provider of innovative solutions for the treatment of severe cardiac diseases, announced that the FDA has approved an Investigational Device Exemption (IDE), allowing the company to begin a pivotal study with i...
Read More...
-Agonist.png)

