Diagnostic Imaging Equipment
Feb 05, 2026
GE HealthCare Secures FDA 510(k) Clearance and CE Mark for Allia Moveo, Completing First Global Installation; China NMPA Approves Promega’s MSI Test as a Companion Diagnostic for KEYTRUDA; Oracle Life Sciences AI Data Platform Unifies Data and Agentic Intelligence to Accelerate Breakthroughs and Boost Commercial Outcomes; Quest Diagnostics Launches New Blood Test for Detecting Minimal Residual Myeloma; RevBio Secures FDA Clearance to Begin Pivotal Study of Cranial Bone Glue; Hyperfine Reports Breakthrough Clinical Findings on Swoop® System’s Increased Sensitivity in Stroke Detection
GE HealthCare Announced the U.S. FDA 510(k) Clearance and CE Mark for Allia Moveo and marks first Global Installation, Advancing Precision Care in the Interventional Suite On February 02, 2026, GE HealthCare announced that Allia™ Moveo received U.S. Food and Drug Administration (FDA) 510(k) clearance and CE Mark...
Read More...
Jan 29, 2026
Seno Medical Secures EU MDR CE Mark for Next-Generation Imagio® Imaging System; Spine Innovation Announces FDA 510(k) Clearance of LOGIC™ Titanium Expandable Interbody System; Laborie Adds JADA® System to Obstetrics Portfolio for Rapid PPH Control; Olympus Introduces SecureFlex™ Single-Use Fine Needle Biopsy Device in the U.S.; Kardium Publishes PULSAR Pivotal Trial Findings in JACC; Myra Vision Begins U.S. ADAPT Glaucoma Study, Treating First Participant
Seno Medical’s Next-Generation Imagio® Imaging System Obtained European Union (EU) Medical Device Regulation (MDR) CE Mark Certification On January 26, 2026, Seno Medical received CE Mark certification for its next-generation Imagio® Imaging System, Model 9100, confirming that the device met the stringent ...
Read More...
Sep 04, 2025
Geneseeq Technology Inc.’s GENESEEQPRIME® Assay Receives FDA 510(k) Clearance; Medtronic’s MiniMed™ 780G Gains FDA Approval for Abbott Instinct Sensor Integration and Type 2 Diabetes Use; CorNeat Vision’s Artificial Cornea Restores 20/20 Vision for Shingles-Induced Blindness; Johnson & Johnson Reports Favorable Outcomes from Real-World VARIPULSE™ PFA Study at 2025 ESC Congress; SprintRay Strengthens Dental 3D Printing Portfolio with EnvisionTEC Acquisition; Olympus Unveils Latest Imaging Platform for ENT and Urology in the U.S.
Geneseeq Technology Inc. Received FDA 510(k) Clearance for GENESEEQPRIME® Assay On September 02, 2025, Geneseeq Technology Inc. announced that the U.S. Food and Drug Administration (FDA) had granted 510(k) clearance for its GENESEEQPRIME® NGS Tumor Profiling Assay. This in vitro diagnostic (IVD) test kit used ne...
Read More...
Aug 21, 2025
FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s Blood Test; Calidar Introduces First-of-its-Kind 4D Mammography System; ONWARD Medical to Initiate Empower BP Pivotal Study Following FDA IDE Approval; Tenon® Medical Launches Catamaran® SE SI Joint Fusion System Nationwide; Rivanna Secures $800,000 Virginia Catalyst Grant Plus Matching Funds to Advance AI-Driven Ultrasound Innovation
Nerveblox, the Ultrasound AI for Regional Anesthesia Received FDA Clearance On August 18, 2025, Nerveblox, an AI-powered software developed by SmartAlpha to assist physicians in performing ultrasound-guided regional anesthesia, received U.S. Food and Drug Administration (FDA) 510(k) clearance. This milesto...
Read More...
Jul 24, 2025
Medtronic Gains CE Mark for MiniMed™ 780G, Begins Onyx™ IDE Study; FDA Clears RIVANNA’s AI Ultrasound Guidance Platform; BD Launches First Clinical Trial with Libertas™ Wearable Injector; Olympus Debuts EU-ME3 Ultrasound Processor in U.S.; Thermo Fisher & Sanofi Expand U.S. Drug Manufacturing Partnership
Medtronic Secured CE Mark Approval for its Minimed™ 780G System, Extending its use to Insulin-Requiring Individuals With Diabetes, Including Children as Young as two Years old, Pregnant Women, and Patients with Type 2 Diabetes On July 21, 2025, Medtronic plc., a global medical technology leader, secured th...
Read More...
Jan 16, 2025
Johnson & Johnson MedTech Secures CE Mark for Dual Energy THERMOCOOL SMARTTOUCH™ SF Catheter; Medtronic Secures CE Mark for BrainSense™ Adaptive Deep Brain Stimulation; Inquis Medical Finalizes Enrollment for U.S. Pivotal Study on Aventus Thrombectomy System for Pulmonary Embolism; First Patient Enrolled in Argon Medical’s CLEAN-PE Study for Novel Pulmonary Embolism Therapy; Sutter Health and GE HealthCare Partner to Revolutionize Patient Care with AI-Driven Imaging Solution; Saluda Medical Closes $100M Financing to Expand Neuromodulation Portfolio
Johnson & Johnson MedTech Announced CE Mark Approval for Dual Energy THERMOCOOL SMARTTOUCHTM SF Catheter, Bolstering Capabilities in Cardiac Arrhythmias Treatment On January 10, 2025, Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, announced the receipt of European CE ma...
Read More...
Jan 09, 2025
Roche Secures FDA Clearance for High-Volume Slide Scanner; Pacira Gets 510(k) Approval for Iovera° SmartTip for Chronic Back Pain; ClearCut Presents Clearcoast™ Study Results at San Antonio Breast Cancer Symposium; NanoVibronix Completes UroShield® Pilot Study at University of Michigan; Hologic Finalizes Acquisition of Gynesonics; Movano Health Launches EvieAI Virtual Wellness Assistant.
Roche's Momentum in Digital Pathology Continued With FDA Clearance on its High-Volume Slide Scanner On January 09, 2025, Roche announced that its whole slide imaging system, Roche Digital Pathology Dx, received an additional 510(k) clearance from the United States Food and Drug Administration (FDA). This c...
Read More...
Dec 19, 2024
Siemens Healthineers Acquires Molecular Imaging Division from Novartis; BD and Babson Diagnostics Unveil Fingertip Blood Testing for Health Care; Medtronic Completes First Ingestion of Pillcam™ Genius SB Kit; FDA Expands Impella Heart Pump Use to Pediatric Patients; Zimmer Biomet Receives FDA Nod for OsseoFit™ Stemless System; enVVeno Medical Completes Final Implants in Pre-Clinical GLP Study
Siemens Healthineers Acquired Novartis’s Advanced Accelerator Applications Molecular Imaging On December 10, 2024, Siemens Healthineers completed the acquisition of Advanced Accelerator Applications Molecular Imaging from Novartis. This European manufacturing and distribution network specializes in diagnos...
Read More...
Dec 05, 2024
FDA Approves Zimmer Biomet’s Oxford® Cementless Partial Knee; ReCerf® Sets Milestone as First All-Ceramic Hip Resurfacing Approved Worldwide; Orthocell Achieves US FDA 510(k) Clearance Milestone for Remplir Regulatory Study; Hologic’s Research Shows AI Breast Imaging Maintains Performance Across All Demographics; GE HealthCare Expands MRI Capabilities with Sonic DL’s Advanced Deep Learning; Terumo Unveils the R2P NaviCross Peripheral Support Catheter
Zimmer Biomet Received FDA Approval for Oxford® Cementless Partial Knee, the Only Cementless Partial Knee Replacement Implant in the U.S. On November 25, 2024, Zimmer Biomet Holdings, Inc., a global leader in medical technology, announced that its Oxford® Cementless Partial Knee received Premarket Approval...
Read More...
Sep 12, 2024
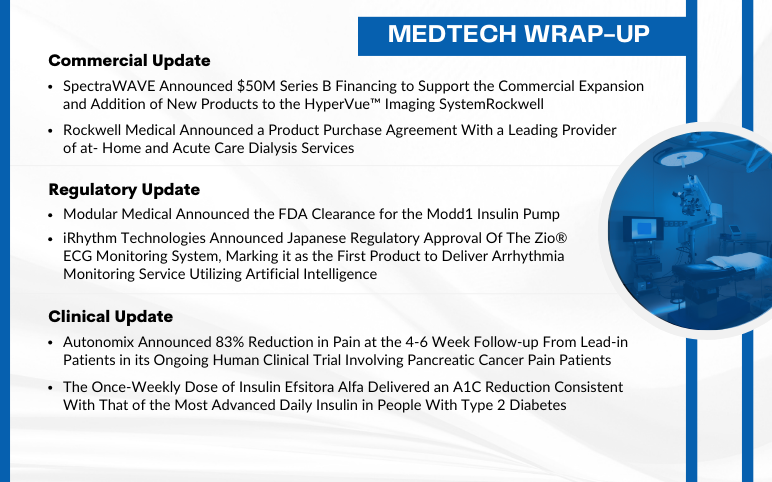
SpectraWAVE raised $50M; Rockwell Medical signed dialysis deal; Modular Medical got FDA nod for Modd1 pump; iRhythm’s Zio approved in Japan; Autonomix trial showed 83% pain reduction; Insulin Efsitora Alfa improved A1C.
Modular Medical Announced the FDA Clearance for the Modd1 Insulin Pump On September 5, 2024, Modular Medical, Inc., an insulin delivery system technology company, announced that it received the U.S. Food and Drug Administration (FDA) clearance to market and sell its MODD1 pump in the United States. With it...
Read More...








-Agonist.png)

