Disposable Endoscopes
Apr 25, 2024
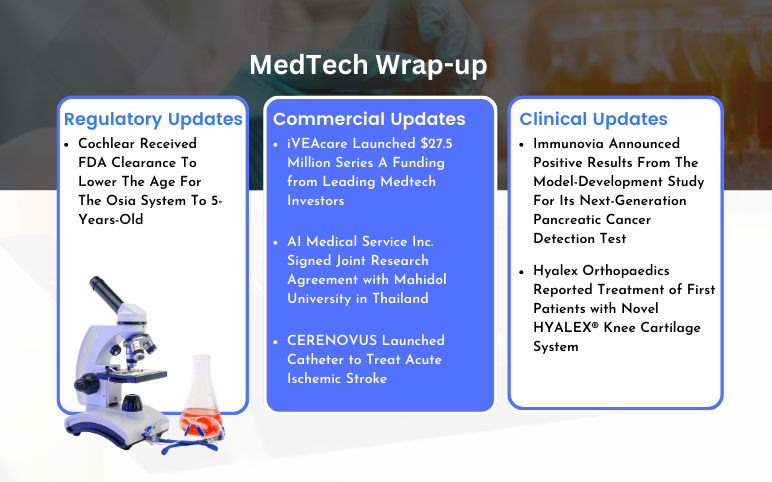
iVEAcare’s $27.5 Million Series A Funding; AI Medical Service Joint Research Agreement with Mahidol University; CERENOVUS Launched Catheter; Cochlear FDA Clearance for The Osia System; Immunovia Announced Positive Results From The Model-Development Study; Hyalex Orthopaedics First Patients Treatment
iVEAcare Launched $27.5 Million Series A Funding from Leading Medtech Investors On April 24, 2024, iVEAcare, announced the closure of a $27.5 million Series A financing. The financing was led by Vensana Capital, which was joined by Treo Ventures, Hatteras Venture Partners, and an undisclosed strategic partner. i...
Read More...
Jul 20, 2023
Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliTec’s Next-Generation EarliPoint Evaluation for ASD; FDA Cleared ReddyPort® Non-Invasive Ventilation Device; EndoTheia’s Novel Medical Device for Endoscopic Surgery; Neurolief’s MOOD Trial Interim Analysis
Relievant Medsystems Launched New Tools for its Ablation System On June 14, 2023, Relievant Medsystems announced that it launched its next-generation access instruments for the Intracept procedure. Chronic vertebrogenic low back pain is treated with Intracept, a minimally invasive, same-day, outpatient proced...
Read More...
Feb 24, 2022
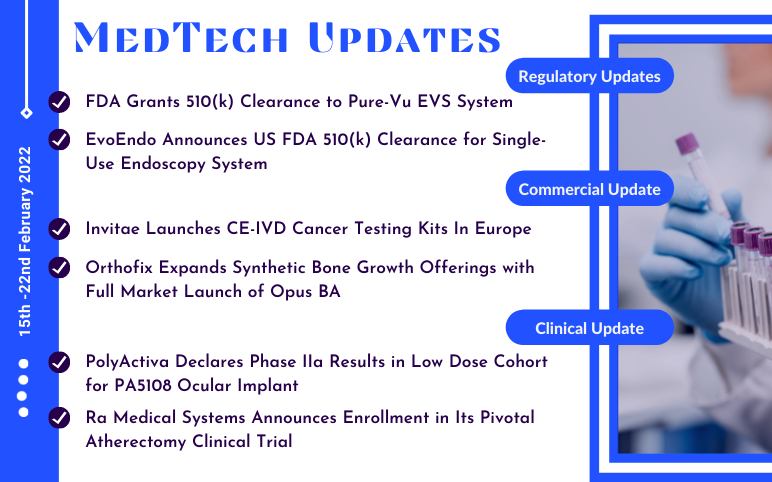
Motus GI’s Pure-Vu EVS System; EvoEndo’s Single-Use Unsedated TNE System; Orthofix’s OpusTM BA; Invitae’s CE-IVD Cancer Testing Kits; PolyActiva’s PA5108 Ocular Implant; Ra Medical’s DABRA excimer laser system
US FDA grants 510(k) clearance to Motus GI’s Pure-Vu EVS System On February 15, 2022, The Pure-Vu® EVS System received 510(k) clearance from the US Food and Drug Administration, according to Motus GI Holdings, Inc., a medical technology company that provides endoscopy solutions that improve clinical outcomes and...
Read More...

-Agonist.png)

