Endoscopes
Jun 19, 2025
Presidio Medical™ Gains IDE Nod for ULF™ Study, Names Dimas Jiménez CFO; Creo Medical Gains FDA Nod for SpydrBlade™ Flex, Paving Way for U.S. Launch; Penumbra Completes Patient Enrollment in Pivotal STORM-PE RCT; Adona Medical Completes Enrollment in First-in-Human Trial of Novel Interatrial Shunt for Heart Failure; Johnson & Johnson Launches TECNIS Odyssey Intraocular Lens in Europe, Middle East, and Canada for Enhanced Cataract Care; Philips Sets New Benchmark in POCUS Technology with Flash 5100 Launch
Presidio Medical™ Received IDE Approval for Ultra Low Frequency (ULF™) Neuromodulation Clinical Study and Appoints Dimas Jiménez as Chief Financial Officer On June 12, 2025, San Mateo, California–Presidio Medical, Inc., a global clinical-stage medical device company, received Investigational New Drug (IDE)...
Read More...
May 29, 2025
Olympus Gains FDA Clearance for EDOF™ Imaging Endoscopes; FDA Approves Abbott’s Tendyne™ Device for Minimally Invasive Mitral Valve Replacement; New Study Validates Exact Sciences’ Oncodetect™ for Enhanced MRD Detection in Stage II-IV Colorectal Cancer; Sensome Completes First-in-Human Trial Enrollment for Lung Cancer Detection System; J&J MedTech Introduces SOUNDSTAR CRYSTAL™ to US Market with Breakthrough 2D Imaging Quality; Terumo Launches ROADSAVER™ Carotid Stent System to Improve Outcomes in Carotid Artery Stenosis
Olympus Secured FDA Clearance for Cutting-Edge EDOF™ Imaging Endoscopes, Redefining Visibility with Sharper, Blur-Free Views On May 27, 2025, Olympus Corporation, a global leader in medical technology and endoscopic imaging, announced that it received FDA 510(k) clearance for its next-generation EZ1500 series en...
Read More...
Jul 04, 2024
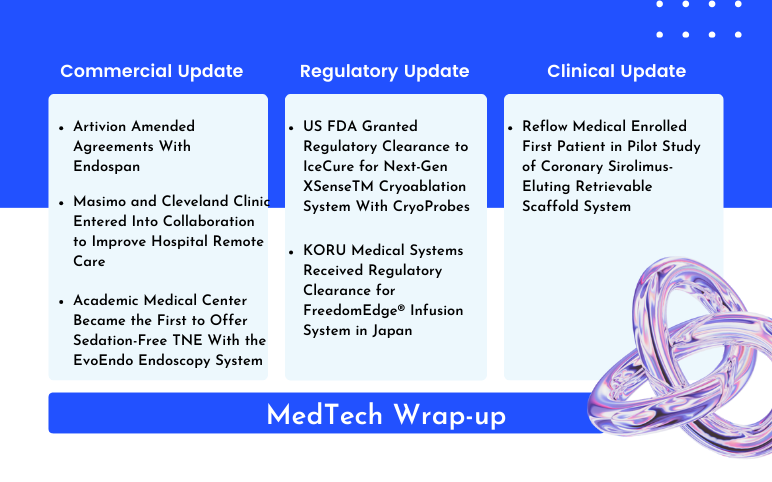
Artivion and Endospan Agreement; Masimo and Cleveland Clinic Collaboration; Academic Medical Center’s Sedation-Free TNE With the EvoEndo Endoscopy System; IceCure’s Next-Gen XSenseTM Cryoablation System FDA Approval; KORU Medical Systems’ Clearance for FreedomEdge® Infusion System; Reflow Medical’s First Patient in Coronary Sirolimus-Eluting Retrievable Scaffold System
Artivion Amended Agreements With Endospan On June 01, 2024, Artivion, Inc. a prominent company in cardiac and vascular surgery specializing in aortic disease, announced that it revised its credit facility and option purchase agreements with Endospan Ltd. ("Endospan"), an Israeli-based, privately-held developer o...
Read More...
Oct 26, 2023
Cardio Flow’s FreedomFlow Orbital Atherectomy Peripheral Platform; Medtronic’s Aurora EV- ICD System to Treat Arrhythmias; GE Healthcare and Novo Nordisk Announces Collaboration; Olympus’s EVIS X1 Endoscopy System; Medtronic’s Evolut TAVR Platform Outperformed Surgery with Sustained Valve Performance; Abbott’s Minimally Invasive Devices for People with Leaky Heart Valves
Olympus Announced Market Launch of EVIS X1™ Endoscopy System On October 19, 2023, Olympus Corporation, a global medical technology company announced the market launch of its next-generation EVIS X1™ endoscopy system. The GIF-1100 gastrointestinal videoscope indicated for use in the upper digestive tract, and ...
Read More...
Aug 04, 2022
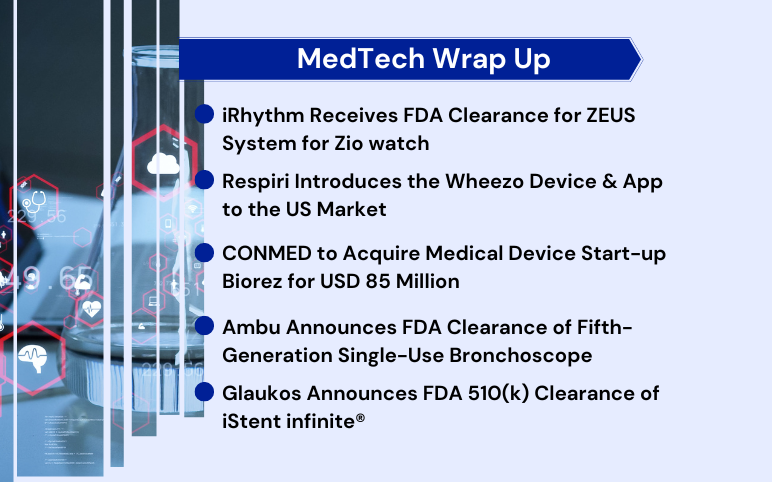
FDA Clearance to iRhythm’s ZEUS System; Respiri Introduces the Wheezo Device & App; CONMED to Acquire Biorez for $ 85 Million; FDA Clearance to Ambu’s Single-Use Bronchoscope; FDA 510(k) Clearance to Glaukos’s iStent infinite
iRhythm receives FDA clearance for ZEUS System for Zio watch iRhythm Technologies, a leading digital healthcare solutions firm focused on advancing cardiac care, announced that it received FDA 510(k) clearance for the ZEUS (Zio ECG Utilization Software) System for the Zio Watch. It is produced in partnership wit...
Read More...
Jul 14, 2022
Zsquare ENT-Flex Rhinolaryngoscope Receives FDA Clearance; ClearMind Biomedical’s Completion of Least Invasive ICH Treatment; Bruker Launched Tool for ‘Long COVID’ Multi-Organ Risk Assessment; EarlySign, Roche Signed Strategic Deal; FDA Clearance for Leva® Pelvic Health System; NeuroOne’s Evo® sEEG Electrode
Zsquare ENT-Flex™ Rhinolaryngoscope, the First High-Performance Single-Use ENT Endoscope of Zsquare received FDA Clearance On July 06, 2022, Zsquare, one of the leading developers of high-performance, single-use endoscopes, received Food and Drug Administration 510K clearance to market its first product, the Zsq...
Read More...




-Agonist.png)

