gilead sciences
Sep 04, 2024
Nonalcoholic Steatohepatitis (NASH): A Growing Epidemic
Obesity and diabetes are a global epidemic contributing to an increasing prevalence of related systemic disorders, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Nonalcoholic Steatohepatitis is a subtype of NAFLD (Nonalcoholic Fatty Liver disease) that is characterized...
Read More...
Sep 04, 2024
Meeting the Unmet: Nonalcoholic Steatohepatitis (NASH)
Nonalcoholic Steatohepatitis (NASH) is a severe form of nonalcoholic fatty liver disease (NAFLD), characterized by liver inflammation and damage due to fat accumulation. Unlike simple fatty liver, NASH can progress to serious conditions such as fibrosis, cirrhosis, and liver failure. This chronic liver disease is i...
Read More...
Dec 27, 2022
Gilead Sciences’ Sunlenca Approval; FDA Approves Roche’s CD20xCD3 Bispecific Antibody Lunsumio; EU Approves AstraZeneca’s Imfinzi Plus Chemo; Pfizer Files Blockbuster Hope Etrasimod for Ulcerative Colitis; FDA Approves Mosunetuzumab for R/F Follicular Lymphoma; FDA Breakthrough Therapy Designation to Adagrasib Plus Cetuximab for KRAS G12C–Mutated Advanced CRC
FDA Approves Gilead Sciences’ Sunlenca Sunlenca, a Gilead Sciences therapy for people with multidrug-resistant (MDR) HIV infection that only needs to be taken twice a year, has received FDA approval for the second time of asking. Sunlenca, which is based on the HIV capsid inhibitor lenacapavir, is intended to be...
Read More...
Mar 16, 2021
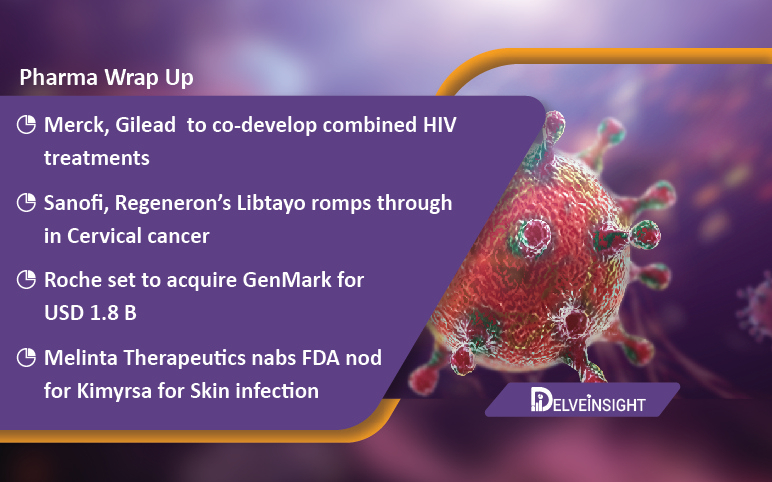
Merck, Gilead teams up for HIV treatment; Bright Future for Sanofi/Regeneron’s Libtayo; Roche acquires GenMark; FDA okays Melinta’s Kimyrsa
Merck, Gilead to co-develop combined HIV treatments Merck and Gilead have announced a collaboration to investigate Gilead’s investigational capsid inhibitor, lenacapavir, and Merck’s investigational nucleoside reverse transcriptase translocation inhibitor, islatravir, into a two-drug regimen. Both...
Read More...
Sep 15, 2020
While AZ resumes its COVID-19 vaccine trial in the UK; Merck and Gilead are busy making sizeable Breast cancer deals
AstraZeneca/ Oxford resumes the trial of their COVID-19 vaccine in the UK After receiving a go-ahead from the UK’s Medicines Health Regulatory Authority, AstraZeneca and the University of Oxford have resumed the trials of their experimental coronavirus candidate AZD1222 in the country. A rapid review by the tria...
Read More...
Aug 13, 2020
Gilead’s remdesivir trials; F2G raises $60.8M; Cancer immunotherapy research updates; Celleron gains rights to Roche’s cancer drug
Researchers call out Gilead over the diversity of remdesivir trials Researchers have challenged the sponsors of COVID-19 clinical trials to run more diverse studies. The call follows an evaluation of Gilead’s remdesivir clinical trials, which found the studies that flunked to offer an equal representation of Bla...
Read More...
Jul 09, 2020
Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks $425M deal; Tregs research updates
Sanofi licenses NK cells of Kiadis to boost the efficacy Sanofi has licensed the natural killer (NK) cells from Kiadis Pharma for use in combination with its multiple myeloma drug Sarclisa. The deal worth of USD 986 million gives the global rights to Sanofi for therapies that could lessen an efficacy-limit...
Read More...
Jun 30, 2020
FDA’s No to Intercept’s NASH Drug; Roche’s Phesgo gets approval; Gilead’s Remdesivir pricing
Intercept Pharmaceuticals receives a clear rejection from the FDA for its NASH drug, Obeticholic acid (OCA) Intercept Pharmaceuticals announced the issuance of a Complete Response Letter (CRL) by the FDA regarding the New Drug Application (NDA) for Obeticholic acid (OCA) for the treatment of fibrosis due to ...
Read More...
Apr 14, 2020
In the fight against COVID-19 – Lilly with Olumiant, Gilead with remdesivir, Rutgers with testing kit
Eli Lilly has announced to begin clinical trials of rheumatoid arthritis drug Olumiant to treat COVID-19 patients. The company plans to explore the anti-inflammatory responses that were witnessed in rheumatoid arthritis patients, and how the drug – Olumiant – an oral JAK1/JAK2 inhibitor – might help in cur...
Read More...
Mar 26, 2020
FDA permits COVID-19 treatment, Roche’s antibiotics pact, Moderna’s vaccine, FDA permits Remdesivir, ReCode needs USD 80 Million
FDA permits COVID-19 treatments with blood from survivors The FDA revealed the convalescent plasma use that is derived from the donated blood of people that have recovered from COVID-19 and that might have beneficial antibodies. It is being used as an investigational treatment for patients with severe cases of t...
Read More...









-Agonist.png)

