Hernia Repair Devices Market
Mar 28, 2024
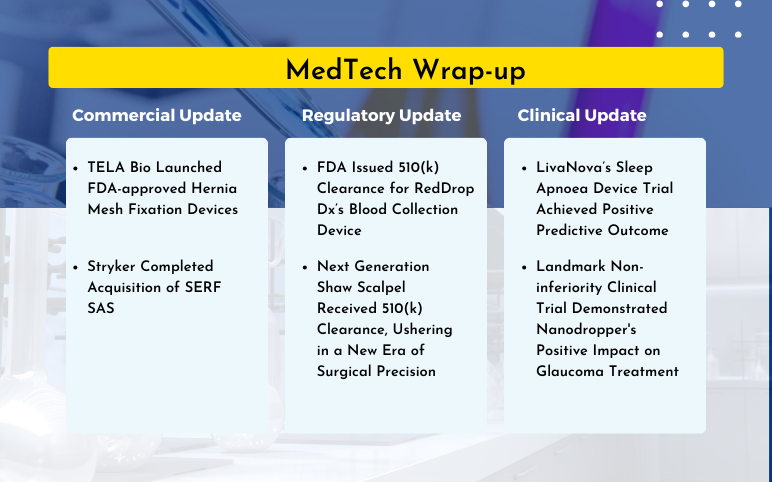
TELA Bio Launched Hernia Mesh Fixation Devices; Stryker Acquisition of SERF SAS; FDA 510(k) Clearance for RedDrop Dx’s Blood Collection Device; FDA 510(k) Clearance for Next Generation Shaw Scalpel; LivaNova’s Sleep Apnoea Device Trial; Nanodropper’s Positive Impact on Glaucoma Treatment
TELA Bio Launched FDA-approved Hernia Mesh Fixation Devices On March 21, 2024, TELA Bio, Inc., a medical technology firm, launched its FDA-approved LIQUIFIX devices for hernia repairs in the United States. The LIQUIFIX FIX8™ and LIQUIFIX Precision™ are specifically engineered for laparoscopic and open femo...
Read More...
Jan 25, 2024
FDA Breakthrough Device Designation to Pi-Cardia’s ShortCut; AbSolutions Med’s REBUILD Bioabsorbable Abdominal Wall Closure Device; AngioDynamics Announces FDA 510(k) Clearance of Auryon XL Radial Access Catheter; Enhatch Announces FDA Clearance for a TKA Patient-Specific Instrumentation System
Pi-Cardia Receives FDA Breakthrough Device Designation for ShortCut™ Pi-Cardia Ltd., a prominent player in advancing catheter-based leaflet modification solutions for heart valve treatment, revealed that its ShortCut™ device has attained Breakthrough Device Designation from the US Food and Drug Administration. S...
Read More...
Dec 08, 2022
Olympus Launches New Gynecologic Power Morcellator; Journey Biosciences Launched NaviDKD; GE Board Approves Separation of GE HealthCare; FDA Approval to Enovis’s STAR Ankle; FDA Clearance to Deep Blue Medical’s T-Line Hernia Mesh; NeuroOne Completed Feasibility Study with its OneRF Ablation System
Olympus Launches New Gynecologic Power Morcellator for Its Contained Tissue Extraction System On November 30, 2022, Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, launched the moresolution™ Power Morcellator, manufactured by TROKAM...
Read More...


-Agonist.png)

