HIV infection market
Jun 30, 2025
Gilead YEZTUGO Makes History with First Twice-Yearly HIV Prevention Drug
Gilead Sciences has received a highly anticipated approval from the FDA for its long-acting HIV prevention injection, YEZTUGO (lenacapavir), marking a significant advancement in HIV prevention. On June 18, 2025, the FDA approved YEZTUGO, an injectable HIV-1 capsid inhibitor, for use as pre-exposure prophylaxis (PrE...
Read More...
Jun 24, 2025
Incyte’s MONJUVI Combo Approved by FDA for Relapsed/Refractory Follicular Lymphoma; Gilead’s YEZTUGO Becomes First FDA-Approved HIV Prevention with 6-Month Protection; FDA Reviewing Incyte’s OPZELURA for Pediatric Atopic Dermatitis (Ages 2–11); Archeus Technologies Receives FDA Clearance for Prostate Cancer Therapy ART-101; Cycle Pharmaceuticals’ HARLIKU Gets First FDA Approval for Alkaptonuria
Incyte’s MONJUVI Combo Approved by FDA for Relapsed/Refractory Follicular Lymphoma Incyte has announced that the FDA has approved MONJUVI (tafasitamab-cxix) in combination with rituximab and lenalidomide for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL). This makes it the f...
Read More...
Mar 16, 2021
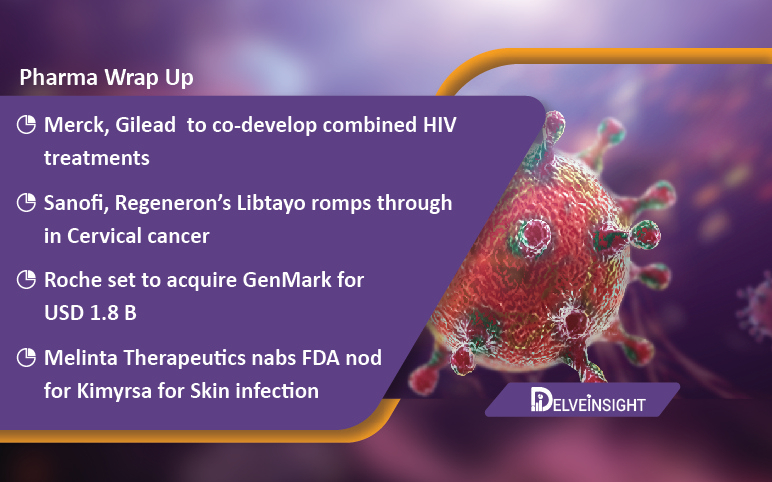
Merck, Gilead teams up for HIV treatment; Bright Future for Sanofi/Regeneron’s Libtayo; Roche acquires GenMark; FDA okays Melinta’s Kimyrsa
Merck, Gilead to co-develop combined HIV treatments Merck and Gilead have announced a collaboration to investigate Gilead’s investigational capsid inhibitor, lenacapavir, and Merck’s investigational nucleoside reverse transcriptase translocation inhibitor, islatravir, into a two-drug regimen. Both...
Read More...


-Agonist.png)

