In Vitro Diagnostics Market
Nov 09, 2023
SIBIONICS’s Ground-breaking GS1 Continuous Glucose Monitoring System; Abbott’s HPV Test to Run on Alinity m; CleanNA’s CE-IVD Molecular Diagnostics Product; Orthofix’s WaveForm L Interbody System; Alucent Biomedical’s AlucentNVS Technology; Surmodics’s TRANSCEND Trial Data
SIBIONICS Received CE Mark for Its Ground-breaking GS1 Continuous Glucose Monitoring System On November 01, 2023, SIBIONICS, the world's third-largest Continuous Glucose Monitoring System (CGM) brand, received the CE Mark for its revolutionary GS1 CGM. This significant milestone marks a momentous achiev...
Read More...
Jun 01, 2023
THINK Surgical’s TMINI Miniature Robotic System; Cepheid’s Xpert NPM1 Mutation Test; Anika’s Hyalofast® US Pivotal Phase III Study; EMVision’s Portable Brain Scanner Trial; HeartBeam and Samsung’s Strategic Alliance Agreement; Eosolutions’s Dr. Banner Balloon Guide Catheter
TMINI Miniature Robotic System by THINK Surgical Received FDA 510(k) Clearance On May 30, 2023, THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its TMINI™ Miniature Robotic System. A wireless r...
Read More...
Apr 06, 2023
Laborie Launched alpHaONE; Cadwell Launched Arc Voyager; Endotronix’s PROACTIVE-HF Pivotal Trial; Sonex Health’s Office-based Carpal Tunnel Release System with Ultrasound Guidance; Leica Biosystems’s BOND MMR Antibody Panel; Abbott Received FDA Approval for Epic™ Max Tissue Valve
Sonex Health Announced US Clinical Study to Report Safety and Effectiveness of Office-based Carpal Tunnel Release System with Ultrasound Guidance On March 30 2023, Sonex Health, Inc., a leader in innovative ultrasound guided therapies to treat common orthopaedic conditions and The Institute of Advanced Ult...
Read More...
Feb 16, 2023
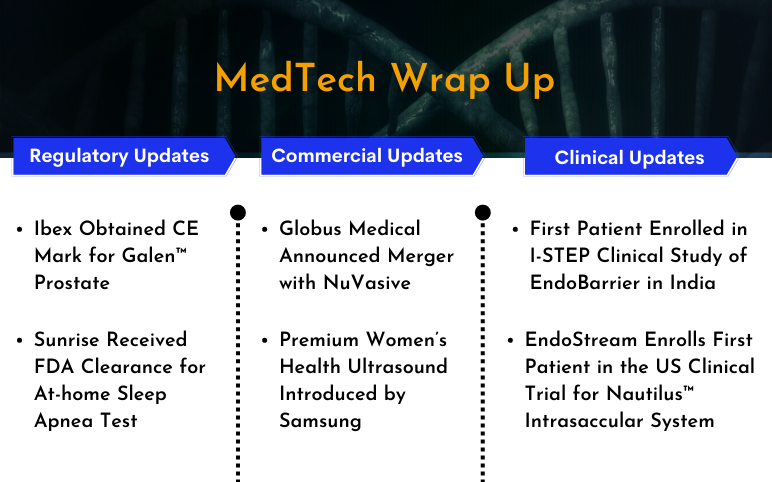
GI Dynamics’s I-STEP Clinical Study of EndoBarrier; EndoStream’s Clinical Trial for Nautilus™ Intrasaccular System; CE Mark for Ibex’s Galen™ Prostate; Sunrise’s At-home Sleep Apnea Test; Globus Medical Announced Merger with NuVasive; Boston Imaging’s HERA W10 Elite
First Patient Enrolled in I-STEP Clinical Study of EndoBarrier in India On February 13, 2023, GI Dynamics Inc., a medical device company, announced the enrollment of the first candidate in the I-STEP clinical trial in India. The trial aimed to assess the safety and effectiveness of the EndoBarrier® System for gl...
Read More...
Dec 22, 2022
Medtronic’s Hugo Robotic-Assisted Surgery System Trial; Insightec’s Pivotal LIBERATE Clinical Trial; FDA Granted EMA for Thermo Fisher Scientific’s Monkeypox Test; Life Spine’s Trulift Lateral Expandable Spacer System & Lateral Pate System; Fujifilm & Inspirata Announces Asset Purchase Agreement; Abbott Launched SCS System for Chronic Pain
Medtronic Announced First Patient Enrolment for Hugo™ Robotic-Assisted Surgery System in US Clinical Trial On December 15, 2022, Medtronic, a global healthcare technology leader, announced that the first patient was enrolled in the Expand URO US clinical trial for the Hugo™ robotic-assisted surgery...
Read More...
Aug 25, 2022
Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to Acquire enCAGE Coil Precision Ablation System; Boston Heart Diagnostics Launches LipidSeqTM; Rocket VR Health Partners with Penn Medicine’s Abramson Cancer Center; Thermedical’s Clinical Trial to Treat Ventricular Tachycardia
Edwards Pascal Precision Transcatheter Mitral And Tricuspid Valve Repair System Receives CE Mark On August 17, 2022, Edwards Lifesciences Corporation, an American medical technology company headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company announc...
Read More...
Jun 02, 2022
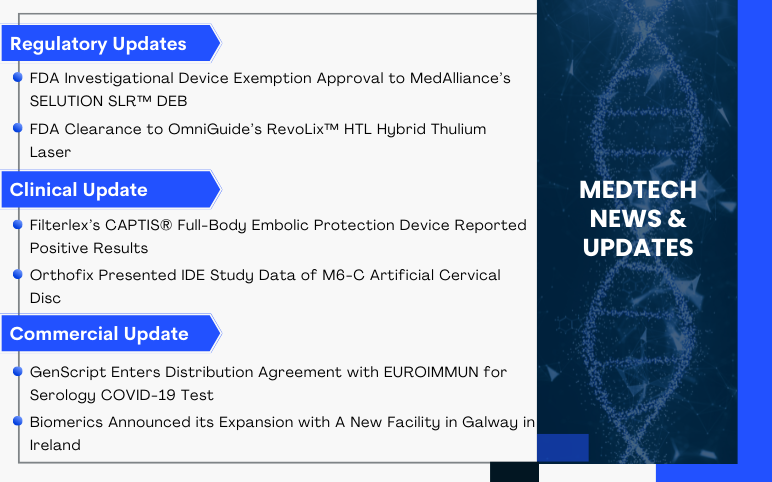
GenScript Enters Distribution Agreement with EUROIMMUN; Biomerics’s New Facility in Galway (Ireland); MedAlliance’s SELUTION SLR DEB; OmniGuide’s RevoLix HTL Hybrid Thulium Laser; Filterlex’s CAPTIS Full-Body Embolic Protection Device; Orthofix’s M6-C Artificial Cervical Disc
GenScript USA Announced the Distribution Agreement with EUROIMMUN US for cPass SARS-CoV-2 Neutralizing Antibody Detection Kit On May 26, 2022, GenScript USA Inc., a subsidiary of GenScript Biotech Corporation and a world-leading biotechnology company, agreed with EUROIMMUN US Inc., a PerkinElmer Company, for the...
Read More...
May 12, 2022
Abbott’s Alinity m STI Assay; Phillips’ MR 7700 System; HOYA’s MiYOSMART Spectacle Lens; Magneto’s Pulmonary Embolism Treatment; Owen Mumford and Stevanto Group’s Collaboration Deal; Elekta’s Radiosurgery System-Elekta Esprit
Abbott Receives Regulatory Approval from US FDA for Alinity™ m STI Assay On May 04, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Alinity™ m STI Assay developed by Abbott which is capable of simultaneously detecting and differentiating four common sexually trans...
Read More...






-Agonist.png)

