DAWNZERA’s EU Approval Strengthens the Hereditary Angioedema Treatment Landscape
Jan 30, 2026
Summary
- The European Commission granted marketing authorization for DAWNZERA (donidalorsen) on January 21, 2026.
- Developed by Ionis Pharmaceuticals and Otsuka Pharmaceutical, it is the first and only RNA-targeted therapy approved in the EU for the prevention of hereditary angioedema (HAE).
- Approval applies to patients aged 12 years and older across the European Union.
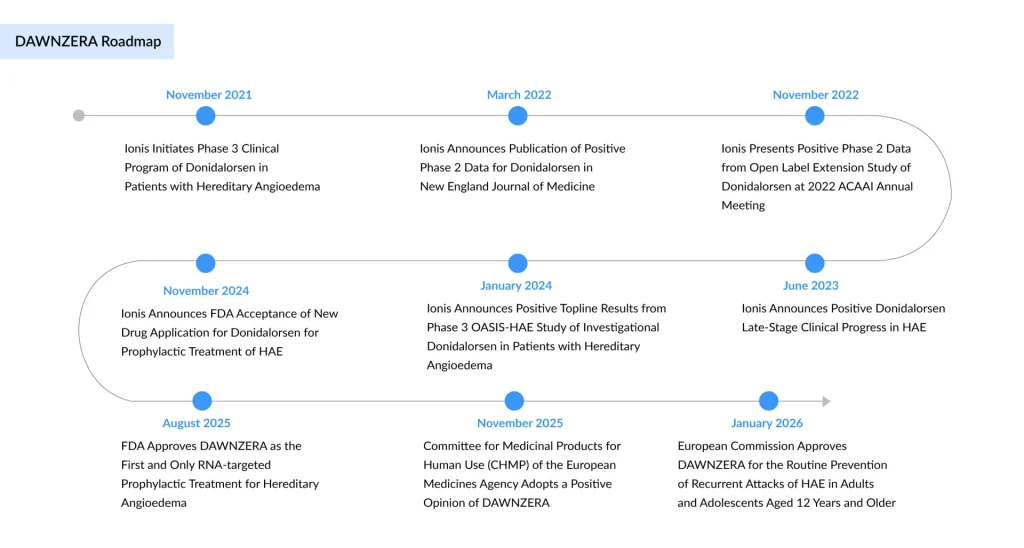
The European Commission has granted marketing authorization for DAWNZERA (donidalorsen), marking a significant milestone in rare disease therapeutics. Developed by Ionis Pharmaceuticals in partnership with Otsuka Pharmaceutical, DAWNZERA is the first and only RNA-targeted therapy for the prevention of hereditary angioedema (HAE) to receive EU approval. The authorization, announced on January 21, 2026, extends this groundbreaking treatment to patients across the European Union aged 12 years and older, addressing a critical unmet clinical need in a patient population historically limited by available prophylactic options.
Hereditary angioedema is a rare yet life-threatening genetic disorder affecting approximately one in 50,000 people worldwide. Characterized by recurrent episodes of severe, unpredictable swelling (angioedema), HAE strikes various body areas, including the hands, feet, genitals, abdomen, face, and throat. The condition results from deficiency or dysfunction of C1-inhibitor (C1-INH), a critical protein that regulates inflammation in the contact system pathway. Without proper control, this dysregulation leads to excessive bradykinin generation, triggering the characteristic swelling attacks that can be debilitating and, in severe cases involving laryngeal involvement, potentially fatal.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Celltrion Announces FDA Nod for New STEQEYMA Presentation to Broaden Pediatric Use; CSL’s ANDEMBR...
- Merck’s WINREVAIR Granted FDA Priority Review for Pulmonary Arterial Hypertension; KalVista’s EKT...
- KalVista’s EKTERLY: First-Ever Oral Hereditary Angioedema Treatment
- BridgeBio bags $299M; Immunochina receives $20M; Attune raises; Wren receives $23M
- Major Highlights and Insights from the AAAAI Annual Meeting, 2024
For decades, HAE patients have faced limited therapeutic options and significant quality-of-life impacts. Acute attacks can be unpredictable and severe, necessitating rapid intervention. The arrival of prophylactic therapies has transformed disease management, yet many patients continue to experience breakthrough attacks or face barriers to treatment access and adherence. DAWNZERA addresses this landscape by offering a novel mechanism of action with demonstrated clinical efficacy.
DAWNZERA operates through a distinct therapeutic mechanism, which distinguishes it from existing HAE treatments. As an RNA-targeted antisense oligonucleotide, donidalorsen specifically targets plasma prekallikrein (PKK), a key enzyme in the contact system cascade that activates inflammatory mediators central to HAE pathophysiology. By reducing plasma prekallikrein production, donidalorsen effectively interrupts the biochemical pathway leading to bradykinin generation and subsequent angioedema attacks.
This targeted approach offers several advantages. First, it addresses a specific molecular driver of disease rather than relying on broad immunosuppressive mechanisms. Second, the RNA-targeting platform enables sustained suppression of the therapeutic target with subcutaneous administration. Third, this mechanism positions DAWNZERA as a distinct option within the prophylactic treatment armamentarium, particularly valuable for patients who may have suboptimal responses to other available agents.
The DAWNZERA approval was supported by favorable data from the Phase 3 OASIS-HAE and OASISplus trials, in which DAWNZERA demonstrated robust efficacy across multiple disease endpoints, including a significant and durable reduction in mean monthly HAE attack rates. In the OASISplus open-label extension, patients achieved a mean reduction in attack rate of 94% at 1 year.
DAWNZERA is administered via a subcutaneous autoinjector every 4 or 8 weeks, offering considerable logistical advantages over intravenous therapies. The subcutaneous route enables patients to self-administer at home, reducing dependence on infusion centers and improving treatment flexibility. The extended dosing interval, compared to the more frequent administration schedules required by some competitors, further enhances real-world convenience and potential adherence.
This administration profile is particularly advantageous in European healthcare systems where outpatient autonomy is increasingly emphasized. For adolescent patients (aged 12–17 years), the autoinjector format removes barriers to self-administration and supports disease self-management during critical developmental periods.
Andy Hodge, President and CEO of Otsuka Pharmaceutical Europe Ltd, emphasized the significance: “We are proud of the decision from the European Commission to authorize the use of donidalorsen in HAE. This represents another key milestone in the collaboration between Otsuka and Ionis, which aims to address unmet needs in a challenging rare disease.”
Industry leaders recognize the strategic importance of DAWNZERA’s EU approval. Henrik Balle Boysen, president of HAE International (HAEi), stated: “As the first European-approved RNA-targeted therapy for HAE, donidalorsen represents a welcome development in therapeutic options for preventing attacks. Today’s approval gives people living with HAE and their physicians another choice for aligning treatment with individual needs.”
DAWNZERA’s European approval followed a well-established regulatory sequence. In November 2025, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion, recommending approval. Following this favorable recommendation, the European Commission granted marketing authorization on January 21, 2026, making DAWNZERA available throughout the EU.
This approval closely followed DAWNZERA’s U.S. FDA authorization in August 2025, underscoring the consistency of regulatory confidence across the world’s two largest pharmaceutical markets. The regulatory trajectory reflects the robust quality of clinical and manufacturing data supporting the application.
Under a partnership framework, Otsuka Pharmaceutical holds exclusive commercialization rights for DAWNZERA across Europe and the Asia-Pacific region. This distribution arrangement leverages Otsuka’s established presence in rare disease markets and commitment to patient access in the EU and adjacent markets.
From Ionis Pharmaceuticals’ perspective, the EU approval triggers significant commercial milestones: the company is eligible for a $15 million milestone payment and tiered royalties of up to 30% on net product sales in the region. These financial terms underscore the commercial significance of the European market and DAWNZERA’s anticipated market penetration.
Brett P. Monia, Chief Executive Officer of Ionis, articulated the strategic vision: “As the first and only RNA-targeted therapy for HAE, we believe DAWNZERA has the potential to become the prophylactic therapy of choice for many patients across the EU.” This positioning reflects confidence in both the clinical evidence and the commercial opportunity within a defined but growing market.
For the estimated thousands of HAE patients across Europe, DAWNZERA’s approval signals meaningful progress in access to effective long-term prophylaxis. The therapy addresses documented barriers to disease control, including limited prophylactic options and the burden of frequent attacks despite existing treatments. The convenient dosing schedule, particularly the quarterly 4-week regimen, may improve treatment adherence and integration into patients’ daily lives.
Furthermore, as regulatory pathways in Europe often influence treatment availability and reimbursement discussions globally, this approval may accelerate discussions on market access and reimbursement in other regions, potentially benefiting HAE patients beyond Europe.
In conclusion, the European Commission’s approval of DAWNZERA represents a watershed moment for hereditary angioedema patients and the rare disease community. By introducing a novel, RNA-targeted mechanism offering exceptional efficacy, 94% mean monthly attack rate reduction, coupled with convenient subcutaneous administration and a favorable safety profile, DAWNZERA addresses genuine unmet clinical needs.
For European patients living with the profound burden of recurrent, unpredictable angioedema attacks, DAWNZERA provides a new option for disease control and improved quality of life. For healthcare systems across the EU, it represents an evidence-based therapeutic innovation in a historically limited therapeutic area. As DAWNZERA reaches patients across Europe, its real-world clinical impact and market penetration will be instructive for future rare disease commercialization strategies.

Downloads
Article in PDF
Recent Articles
- BridgeBio bags $299M; Immunochina receives $20M; Attune raises; Wren receives $23M
- The Expanding Market of Complement Inhibitors
- Major Highlights and Insights from the AAAAI Annual Meeting, 2024
- Merck’s WINREVAIR Granted FDA Priority Review for Pulmonary Arterial Hypertension; KalVista’s EKT...
- KalVista’s EKTERLY: First-Ever Oral Hereditary Angioedema Treatment



