Latest MedTech News
Feb 22, 2024
Johnson & Johnson’s Tecnis PureSee lens; Sparrow BioAcoustics’s Stethoscope Software; Better Therapeutics’s MASH Treatment; X-trodes’ Wearable “Skin” Solution; Lumicell’s LUMISIGHTTM; Konesksa Enrolled First Patient in Learns Observational Study
Johnson & Johnson, Launched New Intraocular Lens in EMEA Region On February 15, 2024, Johnson & Johnson Medtech’s vision unit launched the Tecnis PureSee lens in the EMEA region. Tecnis PureSee is a purely refractive intraocular lens (IOL) specifically designed to correct presbyopia. Its proprietary desi...
Read More...
Feb 08, 2024
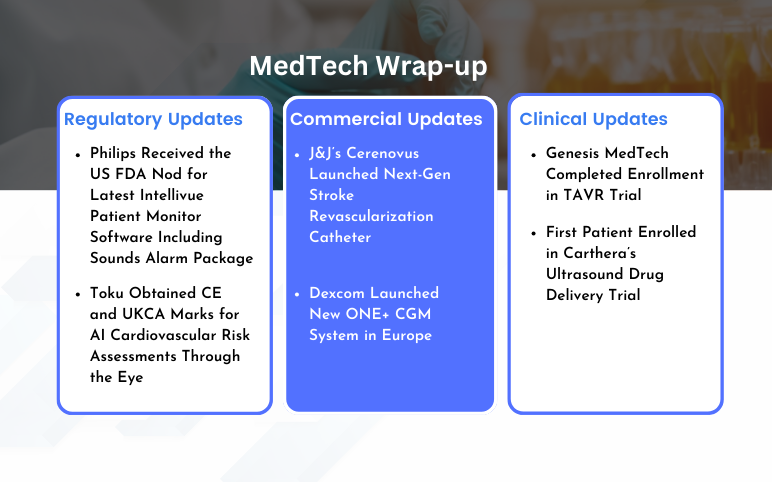
Cerenovus Launched Next-Gen Stroke Revascularization Catheter; Dexcom Launched New ONE+ CGM System; Philips Received the US FDA Nod for Latest Intellivue Patient Monitor Software; Toku Obtained CE and UKCA Marks for AI Cardiovascular Risk Assessments; Genesis MedTech Completed Enrollment in TAVR Trial; Carthera’s Ultrasound Drug Delivery Trial
J&J’s Cerenovus Launched Next-Gen Stroke Revascularization Catheter On February 7, 2024, Johnson & Johnson MedTech’s Cerenovus announced the launch of its next-generation CereGlide 71 intermediate catheter with TruCourse indicated for the revascularization of patients suffering from acute ischemic stroke...
Read More...
Sep 21, 2023
Zepp Health Launched OTC Hearing Aids; Neoss Launched NeoScan 2000; FDA Clearance for AI-Assisted Sleep Monitoring Device Dreem 3S; Tasso Received CE Mark Certification for Tasso+; Neuralink’s First-in-human BCI trial; Creative Medical to Initiate a Phase I/II Clinical Trial of StemSpine
Zepp Health Launched a New Line of OTC Hearing Aids for its Zepp Clarity Brand On September 19, 2023, Zepp Clarity, a smart hearing solutions brand owned by Zepp Health, a health technology company, announced the launch of Zepp Clarity Pixie, a next-generation premium hearing solution. The Pixie, which ...
Read More...
May 25, 2023
Eosolutions Corp’s Dr. Banner Balloon Guide Catheter; US FDA Approves the Cyltezo® Pen; Orlucent’s Handheld Mole Imaging System; Grünenthal’s Resiniferatoxin for Pain Associated with Knee Osteoarthritis; Element Science’s Jewel Patch Wearable Cardioverter Defibrillator; A.Menarini Diagnostics’s PRIME MDx Platform
Eosolutions Announces The Full Commercial Launch of The Dr. Banner Balloon Guide Catheter EOSolutions Corp., a forerunner in medical technology focused on offering high-quality catheter solutions, is pleased to introduce the Dr. Banner, Balloon Guide Catheter (BGC). Dr. Banner, developed in collaboration with In...
Read More...
Mar 30, 2023
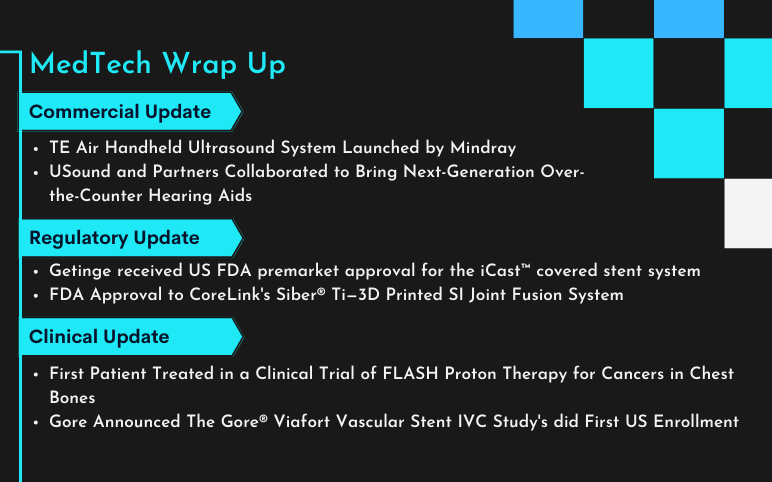
Mindray’s TE Air Handheld Ultrasound System; Getinge’s iCast™ Covered Stent System; Clinical Trial Update for the FLASH Proton Therapy for Cancers in the Bones; Gore’s Gore® Viafort Vascular Stent IVC Study; CoreLink’s Siber® Ti—3D Printed SI Joint Fusion System; USound and Partners’s Next-Generation Over-the-Counter Hearing Aids
TE Air Handheld Ultrasound System Launched by Mindray On March 16, 2023, Mindray, a China-based company announced the launch of TE Air, a high-quality, wireless, handheld, portable ultrasound device that is designed to easily fit into a pocket. Built with Mindray’s eWave platform and second-generation S...
Read More...
Mar 02, 2023
Eko’s AI-powered Sensora Platform; SOPHiA GENETICS and QIAGEN Announced Partnership; Co-Diagnostics’s At-Home and Point-of-Care Co-Dx PCR Home™ Platform; Abbott’s Minimally Invasive Heart Devices Updates; SpectraWAVE’s HyperVue™ Intravascular Imaging System; FDA Approval to Bioelectronic Medicine’s Bioelectric Technology
AI-powered Sensora Platform Launched by Eko for Cardiac Disease Detection On February 23, 2023, Eko, a digital healthcare company employing artificial intelligence (AI) against heart and lung disease, announced the launch of its SENSORA™ Cardiac Disease Detection Platform. The stethoscope, one of the m...
Read More...
Nov 24, 2022
Mainstay Medical’s ReActiv8-C Study; Swing Therapeutics Announced Results from Studies of Stanza; FDA Approves Empatica’s Health Monitoring Platform; FDA Clearance to Persona OsseoTi Keel Tibia System; ClariPi Joins Siemens Healthineers Digital Marketplace; Sony Launches Cloud-based Solution for Flow Cytometry Data Analysis
Mainstay Medical Published Post-Market Clinical Trial Data from Ongoing ReActiv8®-C Study On November 22, 2022, Mainstay Medical, a medical devices company, announced that the data from the ReActiv8®-C study, a single-centre, real-world study with a one-year clinical follow-up of selected patients, is publ...
Read More...
Nov 10, 2022
QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k) Clearance for TriVerse Primary Knee Replacement System; FDA Clearance to ProciseDx’s Reactive Protein (CRP) Assay and ProciseDx Instrument; Evolution Optiks’s Enrollment of LFR-260 Phoropter in US; Kardium First-in-Human Clinical Study of the New Globe Pulsed Field System
QuantuMDx and Menarini Announced an Agreement for the Distribution of the Q-POCTM Platform On November 2, 2022, QuantuMDx Group Limited, a UK-based developer of transformational Point-of-Need molecular diagnostics, and A.Menarini Diagnostics S.r.I. (Menarini), announced an exclusive distribution agreement for Qu...
Read More...
Oct 13, 2022
Neuspera Medical’s Nuvella System; Abbott Presented the Data for FreeStyle Libre 2 System; ZEISS Receives FDA 510(k) Clearance for MTLawton; FDA 510(k) Clearance for the GHOST 3D-Printed Titanium Spacer System; Thermo Fisher Scientific Launched CE-IVD Marked TaqPath Enteric Bacterial Select Panel; BIOCORP and Merck Announced New Partnership
Neuspera Medical Announces First Successful Implant of the Nuvella™ System in the Second Phase of Its Sans-Uui Ide Clinical Trial On October 10, 2022, Neuspera Medical, a medical device company involved in the development of implantable devices for patients suffering from chronic illnesses, announced that...
Read More...
Sep 22, 2022
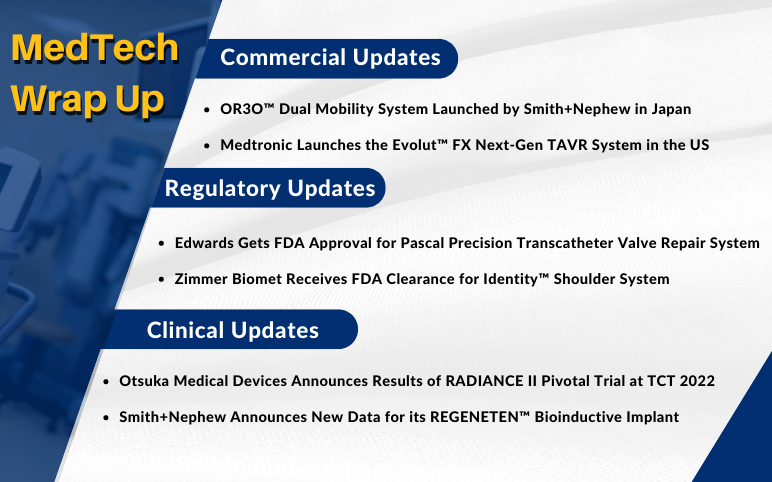
Smith+Nephew’s OR3O Dual Mobility System; Medtronic Launches the Evolut FX Next-Gen TAVR System; FDA Approves Edwards’s Pascal Precision Transcatheter Valve Repair System; FDA Clearance to Zimmer Biomet’s Identity Shoulder System; Otsuka Announces Results of RADIANCE II Trial; Smith+Nephew Announces Data for its REGENETEN™ Bioinductive Implant
OR3O™ Dual Mobility System launched by Smith+Nephew in Japan for use in primary and revision hip arthroplasty On September 20, 2022, Smith+Nephew, a leading global portfolio medical technology business, announced the launch of OR3O Dual Mobility System for use in primary and revision hip arthro...
Read More...







-Agonist.png)

