Micro Invasive Glaucoma Surgery Devices
Jan 29, 2026
Seno Medical Secures EU MDR CE Mark for Next-Generation Imagio® Imaging System; Spine Innovation Announces FDA 510(k) Clearance of LOGIC™ Titanium Expandable Interbody System; Laborie Adds JADA® System to Obstetrics Portfolio for Rapid PPH Control; Olympus Introduces SecureFlex™ Single-Use Fine Needle Biopsy Device in the U.S.; Kardium Publishes PULSAR Pivotal Trial Findings in JACC; Myra Vision Begins U.S. ADAPT Glaucoma Study, Treating First Participant
Seno Medical’s Next-Generation Imagio® Imaging System Obtained European Union (EU) Medical Device Regulation (MDR) CE Mark Certification On January 26, 2026, Seno Medical received CE Mark certification for its next-generation Imagio® Imaging System, Model 9100, confirming that the device met the stringent ...
Read More...
Aug 06, 2025
From Sight to Insight: The Rise of Smart Ophthalmological Devices in Eye Care
Ophthalmology devices are specialized medical instruments and equipment designed for the diagnosis, treatment, and management of eye-related conditions and disorders. These devices encompass a wide range of technologies, including diagnostic tools like optical coherence tomography (OCT) scanners and fundus cameras,...
Read More...
Jul 03, 2025
FDA Clears Stryker’s Incompass Total Ankle System; Glaukos Receives EU MDR Approval for iStent infinite and Key MIGS Devices; STENTiT Announces First Patient Enrollment in Trial of Regenerative Stent for Limb Preservation; Autonomix Medical Treats First Patient in Next Phase (“PoC 2”) of Proof-of-Concept Clinical Trial; Getinge Expands Servo-C Ventilator Capabilities for Neonatal Care; Medtronic Partners With Philips to Advance Patient Monitoring Solutions
Stryker Received FDA Clearance for Incompass Total Ankle System On June 25, 2025, Stryker, a global leader in medical technologies, announced that the U.S. Food and Drug Administration (FDA) received 510(k) approval for its Incompass™ Total Ankle System. The implant is designed for patients suffering from ...
Read More...
Aug 01, 2024
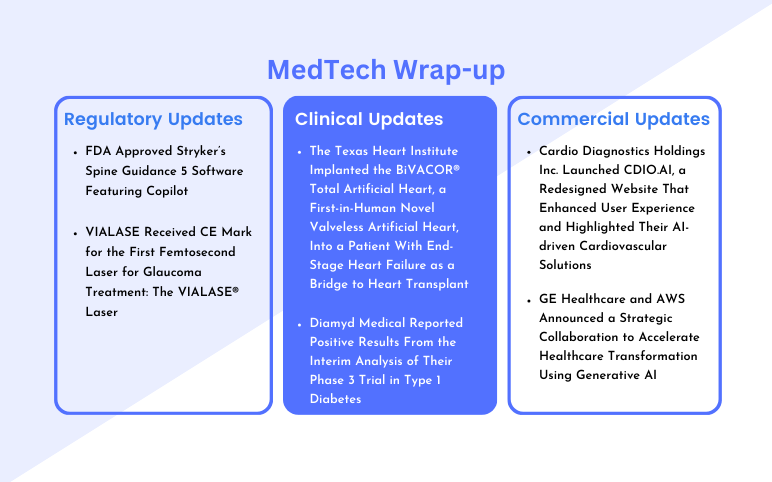
Stryker’s Spine Guidance 5 Software FDA Approval; VIALASE Received CE Mark; The Texas Heart Institute Implanted the BiVACOR® Total Artificial Heart; Diamyd Medical’s Phase 3 Trial Positive Results; Cardio Diagnostics Holdings Inc.’s CDIO.AI; GE Healthcare and AWS Strategic Collaboration
FDA Approved Stryker’s Spine Guidance 5 Software Featuring Copilot On July 30, 2024, Stryker, a global leader in medical technologies, announced that its Q Guidance System with Spine Guidance 5 Software featuring Copilot received 510(k) clearance from the U.S. Food and Drug Administration. This first-to-market t...
Read More...


-Agonist.png)

