Minimally Invasive Surgical (MIS) Devices
Aug 06, 2025
From Sight to Insight: The Rise of Smart Ophthalmological Devices in Eye Care
Ophthalmology devices are specialized medical instruments and equipment designed for the diagnosis, treatment, and management of eye-related conditions and disorders. These devices encompass a wide range of technologies, including diagnostic tools like optical coherence tomography (OCT) scanners and fundus cameras,...
Read More...
Jun 26, 2025
GE HealthCare Receives FDA Approval for Expanded Vizamyl PET Imaging Indications in Alzheimer’s Disease Care; FDA Grants Approval to InspireMD’s CGuard® Prime for Carotid Artery Disease; AngioDynamics Launches RECOVER-AV Trial, Marking Milestone in AlphaVac F18⁸⁵ Development for PE Treatment; Tivic Health Concludes Study Optimizing Non-Invasive Vagus Nerve Stimulation Device for Personalized Therapy; Johnson & Johnson Introduces VOLT™ Plating System and ACUVUE OASYS MAX 1-Day MULTIFOCAL for ASTIGMATISM in the U.S.
GE HealthCare Announced that the FDA Approved Expanded Indications for Vizamyl Pet Imaging Agent for Beta Amyloid Detection, Enabling More Precise Care for Alzheimer’s Patients On June 24, 2025, GE HealthCare announced that the U.S. Food and Drug Administration (FDA) approved an updated label for its PET i...
Read More...
Jan 02, 2025
Genesis Medtech Secures Chinese Approval for Innovative 90° Articulating Stapler; Inogen Secures FDA 510(k) Clearance for SIMEOX 200 Airway Clearance Device; Microtech Unveils First Human Trial of Implantable Microsensor for Heart Failure Monitoring; NAVIGANTIS VASCO™ Platform Begins Clinical Role in Neurovascular Patient Study; Innovent Secures Global Rights from Roche for Novel DLL3 ADC in Exclusive Deal; Treace Expands Portfolio with Percuplasty
Genesis Medtech's World's First 90° Articulation Powered Stapler Achieved Chinese Approval On January 01, 2025, Genesis Medtech announced that iReach Omnia, the world's first powered stapler with 90° articulation capability, received market approval in China from the National Medical Products Administratio...
Read More...
Jul 11, 2024
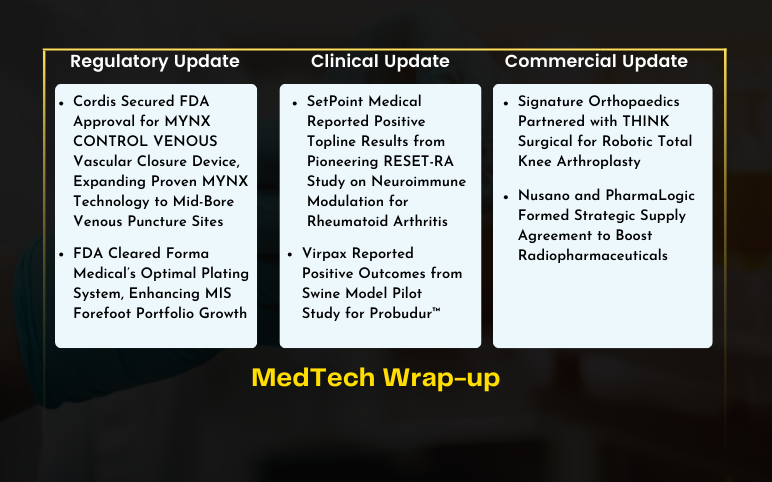
Cordis FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device; Forma Medical’s Optimal Plating System FDA Approval; Virpax’s Swine Model Pilot Study Positive Results; SetPoint Medical Positive Topline Results from Pioneering RESET-RA Study; Signature Orthopaedics Partnered with THINK Surgical; Nusano and PharmaLogic Formed Strategic Supply Agreement
Cordis Secured FDA Approval for MYNX CONTROL VENOUS Vascular Closure Device, Expanding Proven MYNX Technology to Mid-Bore Venous Puncture Sites On July 9, 2024, Cordis, a leading company in cardiovascular and endovascular technology, received FDA approval for its MYNX CONTROL™ VENOUS Vascular Closure Device. Thi...
Read More...
May 23, 2024
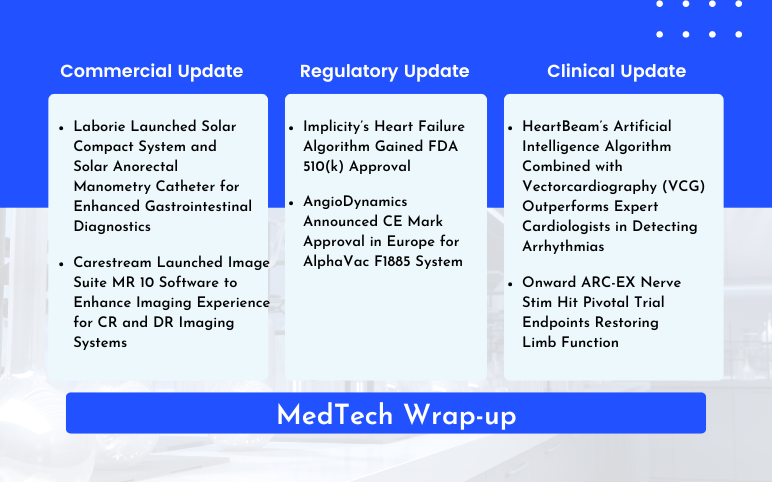
Laborie’s Enhanced Gastrointestinal Diagnostics; Carestream’s Image Suite MR 10 Software Launch; Implicity’s Heart Failure Algorithm FDA 510(k) Approval; AngioDynamics’ AlphaVac F1885 System CE Mark Approval; HeartBeam’s Artificial Intelligence Algorithm for Arrhythmias; Onward ARC-EX Nerve Stim Hit Pivotal Trial
Laborie Launched Solar Compact System and Solar Anorectal Manometry Catheter for Enhanced Gastrointestinal Diagnostics On May 20, 2024, Laborie Medical Technologies Corp., launched the Solar Compact System and Solar Anorectal Manometry Catheter, the first disposable HRAM catheter on the market. These devices sig...
Read More...
Aug 24, 2023
Smith+Nephew’s Hip Arthroplasty System; Mammotome Launched the HydroMARK Plus Breast Biopsy Site Marker; FDA Clearance for Pioneering MARS System; CE Marks for NGS-based Test Kits; BaroPace Updated on Non-Pharmacologic Hypertension and Heart Failure Treatment Trial; Quanta Completed Enrollment in Home Run Study
Smith+Nephew Launched Hip Arthroplasty System in India On August 17, 2023, The London-based orthopedic device maker Smith+Nephew announced the launch of its OR3O dual mobility system for use in primary and revision hip arthroplasty in India. The dual mobility implants have a smaller-diameter femoral hea...
Read More...
Dec 01, 2022
AnchorDx’s UriFind Bladder Cancer Assay in the US; UroMems Initiates Smart Implant to Treat Stress Urinary Incontinence; FDA 510(k) Clearance to NeuroLogica’s BodyTom 64; CE-IVD Mark Approval to SeekInCure’s Recurrence Monitoring Kit Gets; Ypsomed and CamDiab’s Automated Insulin Dosing System; Boston Scientific to Acquire Apollo Endosurgery
AnchorDx Clinical Trial Enrols First Patient for its UriFind® Bladder Cancer Assay in the US On November 23, 2022, AnchorDx, announced the first patient enrollment for its clinical trials of the UriFind® bladder cancer assay in the United States. The UriFind® bladder cancer assay clinical trial in the U...
Read More...
Jul 28, 2022
AbbVie & iSTAR’S Strategic Alliance; Baxter Launches Welch Allyn RetinaVue 100 Imager PRO; Instylla’S Embrace Hydrogel Embolic System; Primary Endpoint Met in the RADIANCE II US Trial; Roche’s Elecsys Amyloid Plasma Panel; EU Approval to Seegene’s Allplex; SARS-CoV-2/FluA/FluB/RSV Assay
ReCor Medical and Otsuka Medical Devices Announce Primary Endpoint Met in the RADIANCE II US Pivotal Trial of the Paradise™ System for the Treatment of Hypertension On July 26, 2022, ReCor Medical, Inc., a completely owned subsidiary of Otsuka Medical Devices Co., Ltd., with its headquarters in Palo Alto,...
Read More...




-Agonist.png)

