Mylan
Aug 30, 2016
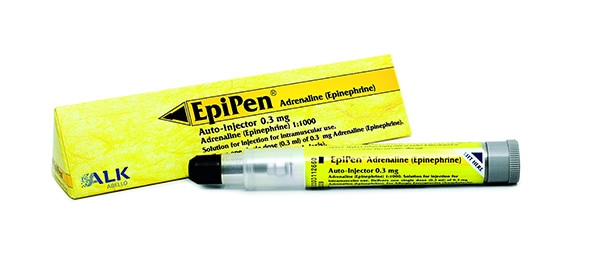
The Road Less Travelled: EpiPen’s Pricing Policy
Mylan’s EpiPen is used as a lifesaver to threatening allergic reactions. It mitigates or reverses allergic reactions including abrupt blocking of airways and swollen legs, chest or neck. In late 2007, Mylan acquired the rights to sell EpiPen, an invention of the 1970s. Even its inventor Sheldon Kaplan did not know ...
Read More...
Aug 25, 2016
NATCO Pharma surges; AstraZeneca falls; US patent office rules
NATCO Pharma surges 7% post successful Establishment Inspection Report for Chennai Plant NATCO pharma has reached a new high of Rs. 703 on the National Stock Exchange (NSE) as the drug maker revealed the successful Establishment Inspection Report (EIR) for Chennai Plant. The U.S. Food and Drug Administration (FDA) h...
Read More...
Jul 18, 2014
Celecoxib first Generic Approval to Teva and Mylan from FDA
On May 30, 2014, the U.S. Food and Drug Administration approved the first generic versions of Celebrex (celecoxib) capsules. Teva Pharmaceutical Industries received approval to market celecoxib capsules in 50 milligram, 100 mg, 200 mg, and 400 mg strengths, and has 180-day exclusivity on the 100 mg, 200 mg, and 400 ...
Read More...

-Agonist.png)

