news
Feb 20, 2024
FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas and Kelonia Enter into Research and License Agreement; Fast Track Designation to Certa’s FT011; Innovent Announces Phase 3 Clinical Trial Updates for IBI311; Orphan Drug Designation to Cardiol’s Pericarditis Drug Candidate
FDA Approves Xolair as First and Only Medicine for Children and Adults with One or More Food Allergies Roche has announced that the FDA has approved Xolair® (omalizumab) to mitigate allergic responses, such as anaphylaxis, that may arise from accidental exposure to various foods in both adult and pediatric patie...
Read More...
Feb 15, 2024
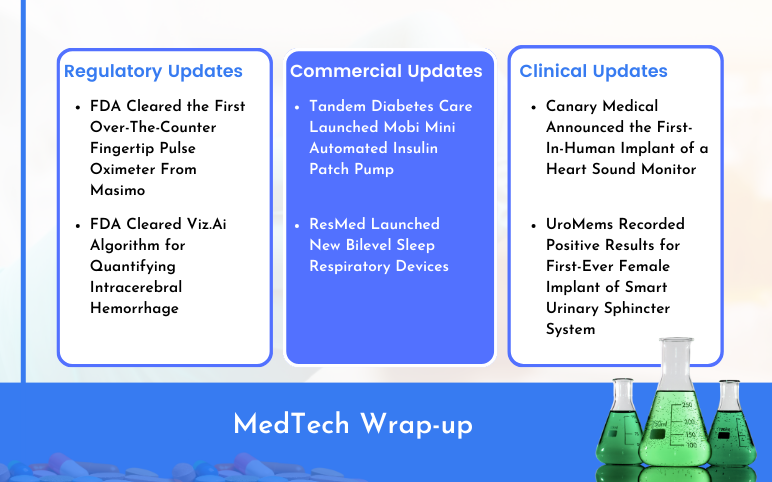
Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First Over-The-Counter Fingertip Pulse Oximeter; Viz.Ai Algorithm for Quantifying Intracerebral Hemorrhage; Canary Medical’s Heart Sound Monitor; UroMems’ Smart Urinary Sphincter System
Tandem Diabetes Care Launched Mobi Mini Automated Insulin Patch Pump On February 13, 2024, announced that it started off the U.S. commercial launch of its Mobi insulin patch pump. The San Diego-based business claims that Mobi, which is fully controllable via a smartphone app, is the world's smallest durable a...
Read More...
Feb 13, 2024
GSK Receives FDA Fast Track Designation for Bepirovirsen; Gilead to Acquire CymaBay Therapeutics; CSL Announces Top-line Results from the Phase III AEGIS-II Trial; Ruxoprubart Scores FDA Orphan Drug Designation for PNH Treatment; CymaBay Announces FDA Acceptance of NDA and Priority Review for Seladelpar; Biogen Received European Commission Approval for SKYCLARYS
GSK Receives FDA Fast Track Designation for Bepirovirsen in Chronic Hepatitis B GSK plc has revealed that the US Food and Drug Administration (FDA) has awarded Fast Track status to bepirovirsen, an experimental antisense oligonucleotide (ASO) designed to treat chronic hepatitis B (CHB). Fast Track designation ai...
Read More...
Feb 07, 2024
Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
As we step into the crisp corridors of 2024, the healthcare landscape unfolds a compelling saga of mergers, strategic funding, and transformative acquisitions. In this month-by-month analysis, we delve into the intricate tapestry of industry dynamics, exploring the impactful maneuvers that are shaping the healthcar...
Read More...
Feb 06, 2024
4DMT Presents Data From Phase II PRISM Clinical Trial; Adaptimmune Announces FDA Acceptance of Biologics License Application for Afami-cel; Astellas Submits Supplemental New Drug Application in Japan for PADCEV with KEYTRUDA; AnaMar Announces US and EU Orphan Drug Designation for AM1476; Biosyngen Announces FDA Fast Track Designation for BST02; Acepodia Announces FDA Clearance of IND Application for ACE2016
4DMT Presents Positive Interim Data from Randomized Phase II PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD 4D Molecular Therapeutics, a prominent company in the field of genetic medicines with a focus on harnessing the full potential of geneti...
Read More...
Feb 01, 2024
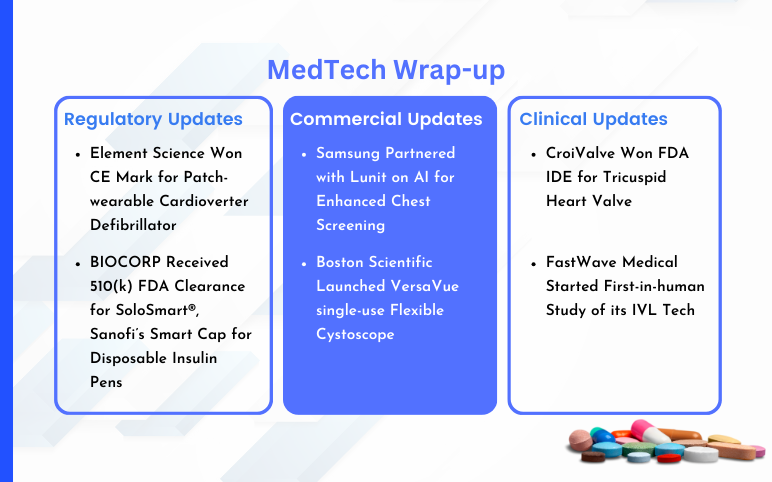
Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope; Element Science’s Patch-wearable Cardioverter Defibrillator; BIOCORP Received 510(k) FDA Clearance for SoloSmart; FDA IDE for CroiValve’s Tricuspid Heart Valve; FastWave Medical’s First-in-human Study of its IVL Tech
Samsung partnered with Lunit on AI for Enhanced Chest Screening On January 25, 2024, Samsung entered into a supply collaboration with Lunit, in order to employ its AI-powered technology for conducting chest screenings. The agreement was signed by Boston Imaging, which serves as the U.S. hub for Samsung's d...
Read More...
Jan 30, 2024
Merck’s KEYTRUDA as Adjuvant Therapy for RCC Patients; BMS Receives Positive CHMP Opinion for CAR T Cell Therapy Abecma for Multiple Myeloma; FDA Approves Dupixent for Eosinophilic Esophagitis; Juvena Receives FDA Orphan Drug Designation for JUV-161; European Commission Authorizes GSK’s Omjjara; ENHERTU Granted Priority Review in the US for for metastatic HER2-positive Solid Tumors
Merck’s KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With Renal Cell Cancer (RCC) at an Increased Risk of Recurrence Following Nephrectomy Merck, also known as MSD beyond the United States and Canada, has revealed findings from the Phase III KEYNOTE-564 trial, which a...
Read More...
Jan 25, 2024
FDA Breakthrough Device Designation to Pi-Cardia’s ShortCut; AbSolutions Med’s REBUILD Bioabsorbable Abdominal Wall Closure Device; AngioDynamics Announces FDA 510(k) Clearance of Auryon XL Radial Access Catheter; Enhatch Announces FDA Clearance for a TKA Patient-Specific Instrumentation System
Pi-Cardia Receives FDA Breakthrough Device Designation for ShortCut™ Pi-Cardia Ltd., a prominent player in advancing catheter-based leaflet modification solutions for heart valve treatment, revealed that its ShortCut™ device has attained Breakthrough Device Designation from the US Food and Drug Administration. S...
Read More...
Jan 23, 2024
BMS, and Exelixis’s Opdivo + CABOMETYX in First-Line Advanced Renal Cell Carcinoma; AIRSUPRA Now Available as the First and Only FDA-approved Anti-inflammatory Rescue Option for Asthma; AstraZeneca’s Voydeya Receives First-ever Regulatory Approval; EMA Grants ODD to GC Biopharma’s Sanfilippo Syndrome (Type A) Treatment; FDA Approves NRx Pharma’s IND Application of NRX-101; FDA Fast Track Designation to Kyverna’s KYV-101
Opdivo in Combination with CABOMETYX Demonstrates Long-Term Survival Benefits After Four Years of Follow-Up in the CheckMate -9ER Trial in First-Line Advanced Renal Cell Carcinoma Bristol Myers Squibb and Exelixis, Inc. have released the four-year follow-up findings from the CheckMate -9ER trial, which investiga...
Read More...
Jan 17, 2024
A Glance at Key Insights From 42nd J.P. Morgan Annual Healthcare Conclave
From January 8th to 11th, 2024, the 42nd Annual J.P. Morgan Healthcare Conference (JPM24) took center stage in San Francisco, CA, USA. Spanning four dynamic days, this conference saw the active participation of prominent figures from major pharmaceutical, biotechnology, Medtech, HealthTech entities, and emerging fa...
Read More...








-Agonist.png)

