news
Dec 13, 2022
Amgen to Purchase Horizon Therapeutics; IND Clearance to Vertex’s VX-522; FDA Fast-Track Designation for Moleculin’s WP1122; Orphan Drug Designation to Brim’s WP1122; Eisai Presents Results of Lecanemab Phase 3 Confirmatory Clarity Ad Study; UCB Announces Phase 3 Studies for Bimekizumab
Amgen Inc. Agrees to Purchase Dublin-based Horizon Therapeutics Plc. for €24.7 Billion Amgen Inc. has agreed to buy Dublin-based Horizon Therapeutics Plc. for €24.7 billion ($26 billion), in a deal that could face further delays or a breakdown in negotiations. Following Sanofi's withdrawal from the race, c...
Read More...
Dec 08, 2022
Olympus Launches New Gynecologic Power Morcellator; Journey Biosciences Launched NaviDKD; GE Board Approves Separation of GE HealthCare; FDA Approval to Enovis’s STAR Ankle; FDA Clearance to Deep Blue Medical’s T-Line Hernia Mesh; NeuroOne Completed Feasibility Study with its OneRF Ablation System
Olympus Launches New Gynecologic Power Morcellator for Its Contained Tissue Extraction System On November 30, 2022, Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, launched the moresolution™ Power Morcellator, manufactured by TROKAM...
Read More...
Dec 06, 2022
Nkarta’s Anti-CD19 Allogeneic CAR-NK Cell Therapy, NKX019; Eisai Presents Results of lecanemab for Alzheimer’s Disease; EQRx’s Aumolertinib for EGFR-Mutated NSCLC; FDA Approves Olutasidenib for IDH1-Mutated R/R AML; FDA Orphan Drug Designation to AUM302 for Neuroblastoma; X4 Pharma Announces Results for WHIM Syndrome Drug
FDA Grants Orphan Drug Designation to AUM302 for Neuroblastoma A global clinical-stage biotech company, AUM Biosciences, focused on discovering and developing precision oncology therapeutics, declared that the U.S. FDA has permitted Orphan Drug Designation for AUM302. For the treatment of neuroblastoma, AUM302 i...
Read More...
Dec 01, 2022
AnchorDx’s UriFind Bladder Cancer Assay in the US; UroMems Initiates Smart Implant to Treat Stress Urinary Incontinence; FDA 510(k) Clearance to NeuroLogica’s BodyTom 64; CE-IVD Mark Approval to SeekInCure’s Recurrence Monitoring Kit Gets; Ypsomed and CamDiab’s Automated Insulin Dosing System; Boston Scientific to Acquire Apollo Endosurgery
AnchorDx Clinical Trial Enrols First Patient for its UriFind® Bladder Cancer Assay in the US On November 23, 2022, AnchorDx, announced the first patient enrollment for its clinical trials of the UriFind® bladder cancer assay in the United States. The UriFind® bladder cancer assay clinical trial in the U...
Read More...
Nov 29, 2022
C4X Discovery and AstraZeneca Signs Deal; FDA Rejects Spectrum’s Poziotinib; Orphan Drug Designation to Tenaya’s Gene Therapy; EC Approves Regeneron’s Libtayo; Response Letter to Poziotinib for Metastatic NSCLC Harboring HER2 Exon 20 Mutations; Japan Approves Trastuzumab Deruxtecan for HER2+ Breast Cancer
C4X Discovery Holdings and AstraZeneca Signs Exclusive USD 402 Million Global License C4X Discovery Holdings has signed an exclusive global license with AstraZeneca worth up to USD 402 million for the development and commercialization of the NRF2 Activator program. The agreement will allow AstraZeneca to develop...
Read More...
Nov 24, 2022
Mainstay Medical’s ReActiv8-C Study; Swing Therapeutics Announced Results from Studies of Stanza; FDA Approves Empatica’s Health Monitoring Platform; FDA Clearance to Persona OsseoTi Keel Tibia System; ClariPi Joins Siemens Healthineers Digital Marketplace; Sony Launches Cloud-based Solution for Flow Cytometry Data Analysis
Mainstay Medical Published Post-Market Clinical Trial Data from Ongoing ReActiv8®-C Study On November 22, 2022, Mainstay Medical, a medical devices company, announced that the data from the ReActiv8®-C study, a single-centre, real-world study with a one-year clinical follow-up of selected patients, is publ...
Read More...
Nov 22, 2022
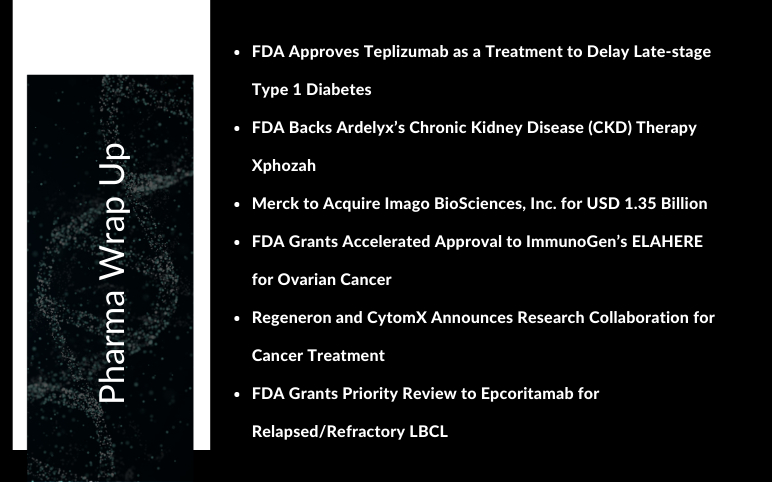
FDA Approves Teplizumab to Delay the Onset of Type 1 Diabetes; FDA Backs Ardelyx’s CKD Therapy Xphozah; Merck to Acquire Imago BioSciences; Accelerated Approval to ImmunoGen’s ELAHERE; Regeneron and CytomX Announces Research Collaboration; FDA Grants Priority Review to Epcoritamab R/R LBCL
Merck to Acquire Imago BioSciences, Inc. for USD 1.35 Billion While its rumored acquisition of Seagen has yet to materialize, Merck & Co has continued to bolster its pipeline with smaller transactions, the most recent of which was a USD 1.35 billion agreement to acquire Imago BioSciences and its bomedemstat ...
Read More...
Nov 17, 2022
Medtronic Launches Infusion Set for Insulin Pumps; Penumbra’s Virtual Reality-Based Rehabilitation System; FDA Approval to Genesis’s Chocolate Touch® Drug-coated Balloon PTA Catheter; FDA Nod to AEYE’s AI-based Autonomous Screening; Alpheus Medical Treats First High-Grade Glioma Brain Cancer Patient with its Proprietary Platform; Karidum’s Next Generation Globe® Pulsed Field System
Medtronic Launches World's First and Only Infusion Set for Insulin Pumps that Doubles Wear Time up to 7 days in the US On November 15, 2022, Medtronic plc, a global leader in healthcare technology, announced the US launch of the Medtronic Extended infusion set, the first and only infusion set labeled for up to ...
Read More...
Nov 15, 2022
Genentech’s gantenerumab Fails in Phase III Trial; CHMP Recommends’ Dupixent; FDA Clears Imfinzi and Imjudo with chemotherapy; NICE Recommends Ipsen’s Cabometyx (cabozantinib); NICE Backs KEYTRUDA; NRG Announces £16 Million Series A Funding; FDA Backs AstraZeneca’s PT027
Genentech’s gantenerumab Fails in Phase III Trial for Alzheimer’s Disease In yet another setback for an amyloid-targeting drug, Roche's Genentech division has reported disappointing top-line results from its highly anticipated phase III trial of gantenerumab in early Alzheimer's disease. The failure is entirely ...
Read More...
Nov 10, 2022
QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k) Clearance for TriVerse Primary Knee Replacement System; FDA Clearance to ProciseDx’s Reactive Protein (CRP) Assay and ProciseDx Instrument; Evolution Optiks’s Enrollment of LFR-260 Phoropter in US; Kardium First-in-Human Clinical Study of the New Globe Pulsed Field System
QuantuMDx and Menarini Announced an Agreement for the Distribution of the Q-POCTM Platform On November 2, 2022, QuantuMDx Group Limited, a UK-based developer of transformational Point-of-Need molecular diagnostics, and A.Menarini Diagnostics S.r.I. (Menarini), announced an exclusive distribution agreement for Qu...
Read More...








-Agonist.png)

