Peripheral T-cell Lymphoma
Jun 25, 2024
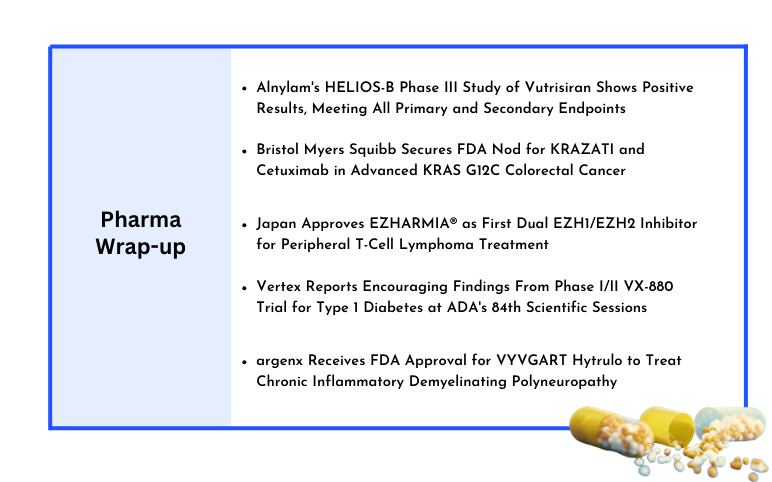
Alnylam’s HELIOS-B Phase III Study of Vutrisiran; Bristol Myers Squibb Secures FDA Nod for KRAZATI and Cetuximab; Daichii Sankyo’s EZHARMIA® Receives Japan Approval; Vertex’s Phase I/II VX-880 Trial; argenx’s VYVGART Hytrulo FDA Approval
Alnylam's HELIOS-B Phase III Study of Vutrisiran Shows Positive Results, Meeting All Primary and Secondary Endpoints Alnylam Pharmaceuticals, Inc. reported encouraging topline outcomes from its HELIOS-B Phase III study of vutrisiran, an experimental RNAi therapy being developed to treat ATTR amyloidosis with car...
Read More...
Sep 19, 2023
FDA Approves Ojjaara for Myelofibrosis; EMA Grants PRIME Designation to Iopofosine I-131; EBGLYSS Receives Positive CHMP Opinion; FDA Accepts Resmetirom NDA; FDA Fast Track Designation to KT-333 for PTCL; RedHill Announces FDA sNDA Approval for Talicia®
EBGLYSS Receives Positive CHMP Opinion for Moderate-to-Severe Atopic Dermatitis Almirall S.A. announced that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion recommending the marketing authorization of EBGLYSS (lebrikizumab) for the treatment o...
Read More...
Jan 14, 2022
Most Promising Oncological Drugs Expected to Launch in 2022
The innovation in the oncology drug pipeline has resulted in a record number of FDA and EU approvals in recent years, as investigators and sponsors seek new and targeted treatments for individuals diagnosed with different types of cancers each year. In 2022, regulators will continue to evaluate new oncology therapi...
Read More...


-Agonist.png)

