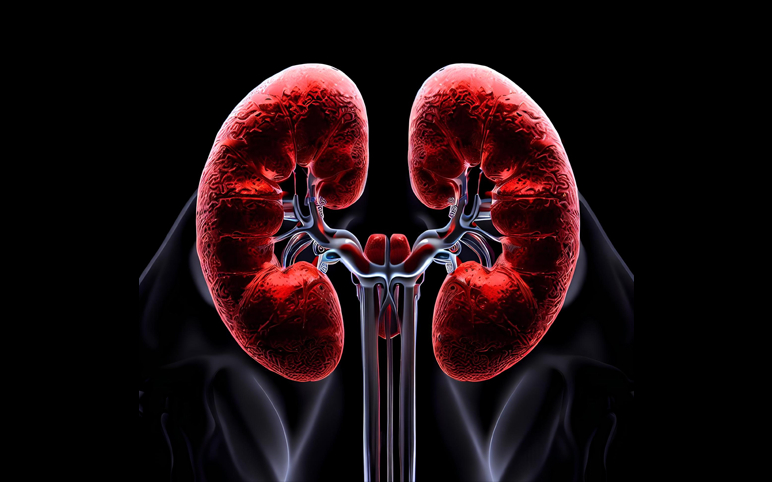
Primary Hyperoxaluria
Oct 09, 2023
Can Novo’s Rivfloza Outperform Its Rival Alnylam’s Oxlumo in Primary Hyperoxaluria Treatment Space?
In recent years, Novo Nordisk has been actively addressing the challenges arising from the launch of its highly popular obesity therapy, Wegovy. However, the pharmaceutical company is now venturing into a less common medical territory with its latest FDA approval. Novo has received FDA approval for Rivfloza, a trea...
Read More...
Oct 03, 2023
AbbVie Presents Phase III CANOVA Study Results; Novartis’ Iptacopan Shows Promise in Phase III Study; Fast Track Designation to AVB-001 for R/R Platinum-Resistant Ovarian Cancer; FDA Issues Complete Response Letter for Lebrikizumab; Nedosiran Approveed for Primary Hyperoxaluria Type 1; Orphan Drug Designation to BDC-1001 for Gastric Cancers
AbbVie Presents Results from Phase III CANOVA Study of Venetoclax in Patients with Relapsed or Refractory Multiple Myeloma AbbVie has released findings from its Phase III CANOVA trial, which assessed the safety and effectiveness of venetoclax (marketed as VENCLEXTA®/VENCLYXTO®) in combination with dexamethasone ...
Read More...
Aug 09, 2021
Primary Hyperoxaluria Market Size Observes Growth with a Substantial CAGR
Primary hyperoxaluria (PH) is a rare autosomal genetic form of Hyperoxaluria, a condition that leads to the excessive urinary excretion of oxalate. Till now, three distinct hereditary enzymatic deficiencies have been identified as a cause of PH, namely, PH type 1 (PH1), type 2 (PH2), and type 3 (PH3), with PH1 as t...
Read More...


-Agonist.png)

