Sleep Apnea Devices
Jun 12, 2025
Samsung Galaxy Watch Sleep Apnea Feature Cleared for Europe With CE Certification; ZEISS CLARUS 700 Gains NMPA Approval in China; Envoy Medical’s Acclaim® Cochlear Implant Trial Progresses as Planned Following First Month Follow-Up; Masimo SedLine® Shown to Enhance Brain Monitoring Accuracy in Pediatric Anesthesia; Penumbra Launches Ruby® XL System; Johnson & Johnson Introduces the ETHICON™ 4000 Stapler
Samsung Expands Global Reach of Sleep Apnea Feature on Galaxy Watch With CE Marking Certification in Europe On June 5, 2025, Samsung Electronics Co., Ltd. announced the expanded global availability of its Sleep Apnea feature on the Galaxy Watch series, accessible through the Samsung Health Monitor app. The...
Read More...
May 08, 2025
Teleflex Receives FDA 510(k) Clearance to Expand Use of QuikClot Control+™; Edwards Wins FDA Nod for TAVR Use in Asymptomatic Aortic Stenosis; LivaNova Announces Positive 12-Month Results from OSPREY Study in Moderate to Severe OSA; Lipogems Completes Final Patient Visit in ARISE 1 Knee Osteoarthritis Trial; Roche Launches Advanced Elecsys PRO-C3 Test for More Accurate Liver Fibrosis Grading; iRhythm Introduces Zio® Long-Term ECG Monitoring in Japan, Enhancing AI-Driven Arrhythmia Detection
Teleflex Secures FDA 510(k) Clearance for Broader Use of QuikClot Control+™ Hemostatic Device On April 30, 2025, Teleflex Incorporated, a global leader in medical technologies, announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the expanded Indications for Use ...
Read More...
Sep 26, 2024
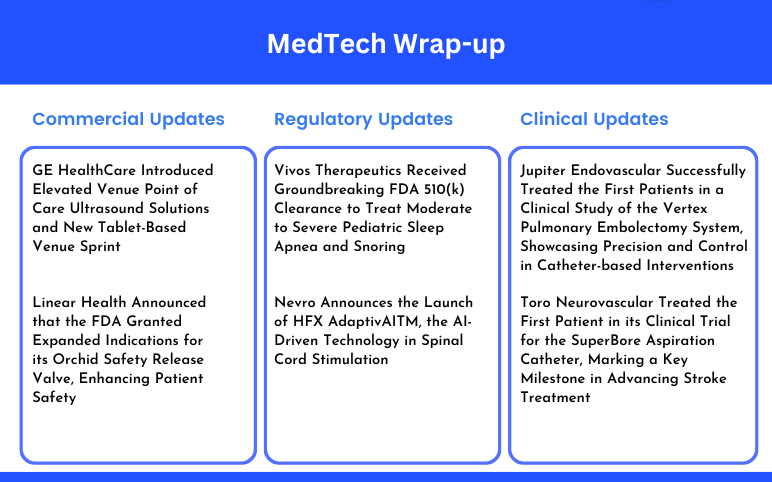
Vivos Therapeutics Receives FDA 510(k) Clearance for Pediatric Sleep Apnea; Nevro Launches AI-Driven Spinal Cord Stimulation; Jupiter Endovascular Treated First Patients in Pulmonary Embolectomy System Study; Toro Neurovascular Advances Stroke Treatment with SuperBore Aspiration Catheter; GE HealthCare Introduces Venue Ultrasound Solutions; Linear Health Expands Orchid Safety Valve Indications
Vivos Therapeutics Received Groundbreaking FDA 510(k) Clearance to Treat Moderate to Severe Pediatric Sleep Apnea and Snoring On September 18, 2024, Vivos Therapeutics, Inc., a premier medical device and technology firm focused on innovative treatments for sleep-related breathing disorders (SRBDs), including obs...
Read More...
Feb 15, 2024
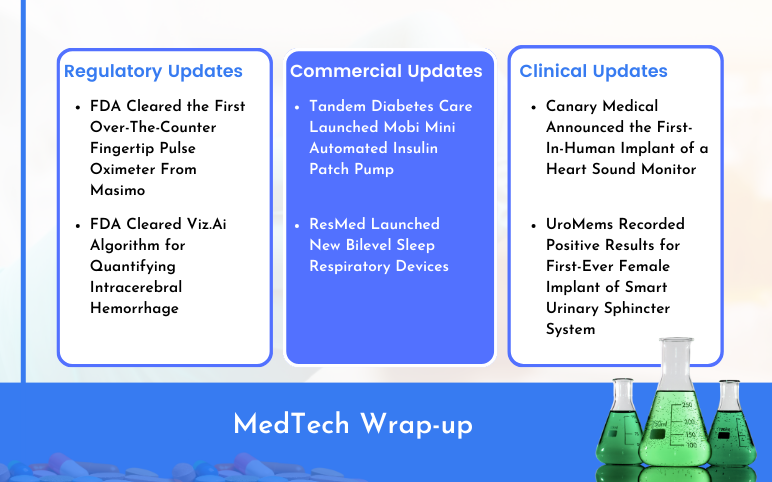
Tandem’s Automated Insulin Patch Pump; ResMed’s Bilevel Sleep Respiratory Devices; Masimo’s First Over-The-Counter Fingertip Pulse Oximeter; Viz.Ai Algorithm for Quantifying Intracerebral Hemorrhage; Canary Medical’s Heart Sound Monitor; UroMems’ Smart Urinary Sphincter System
Tandem Diabetes Care Launched Mobi Mini Automated Insulin Patch Pump On February 13, 2024, announced that it started off the U.S. commercial launch of its Mobi insulin patch pump. The San Diego-based business claims that Mobi, which is fully controllable via a smartphone app, is the world's smallest durable a...
Read More...
Dec 07, 2023
Johnson & Johnson Medtech Acquired Laminar; BD Launched Advanced Vascular Access Ultrasound System; FDA Cleared Oral Device for Severe Sleep Apnea; FDA Clearanced the Zilia OcularTM FC Retinal Camera; First Patient Enrolled in Penumbra Study of Computer-assisted Vacuum Thrombectomy; US FDA Granted the BiVACOR Total Artificial Heart IDE Approval for First-in-Human Early Feasibility Study
FDA Cleared Oral Device for Severe Sleep Apnea From Vivos Therapeutics On November 29, 2023, Vivos Therapeutics announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Vivos’s removable CARE (Complete Airway Repositioning and Expansion) oral appliances developed for treat...
Read More...



-Agonist.png)

