Spinal Cord Stimulators
Sep 11, 2025
ONWARD Medical’s ARC-EX System Gains CE Mark; Microbot Medical Secures FDA 510(k) Clearance for LIBERTY® Endovascular Robotic System; Fraiya Launches Major NHS Clinical Trial Using AI for Pregnancy Ultrasounds; Medtronic Hugo™ Robotic-assisted Surgery System in Hernia Repair Met Safety and Effectiveness Endpoints; Willow Launches Willow Wave, Pioneering a New Standard in Breastfeeding; Vivalink Launches New Adhesive to Improve Wearable ECG Performance
ONWARD Medical Received CE Mark for ARC-EX, Enabling Commercial Launch of Breakthrough Spinal Cord Stimulation System in Europe On September 8, 2025, ONWARD Medical N.V., a leading neurotechnology company developing therapies to restore movement, function, and independence for individuals with spinal cord ...
Read More...
Aug 21, 2025
FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s Blood Test; Calidar Introduces First-of-its-Kind 4D Mammography System; ONWARD Medical to Initiate Empower BP Pivotal Study Following FDA IDE Approval; Tenon® Medical Launches Catamaran® SE SI Joint Fusion System Nationwide; Rivanna Secures $800,000 Virginia Catalyst Grant Plus Matching Funds to Advance AI-Driven Ultrasound Innovation
Nerveblox, the Ultrasound AI for Regional Anesthesia Received FDA Clearance On August 18, 2025, Nerveblox, an AI-powered software developed by SmartAlpha to assist physicians in performing ultrasound-guided regional anesthesia, received U.S. Food and Drug Administration (FDA) 510(k) clearance. This milesto...
Read More...
Jan 16, 2025
Johnson & Johnson MedTech Secures CE Mark for Dual Energy THERMOCOOL SMARTTOUCH™ SF Catheter; Medtronic Secures CE Mark for BrainSense™ Adaptive Deep Brain Stimulation; Inquis Medical Finalizes Enrollment for U.S. Pivotal Study on Aventus Thrombectomy System for Pulmonary Embolism; First Patient Enrolled in Argon Medical’s CLEAN-PE Study for Novel Pulmonary Embolism Therapy; Sutter Health and GE HealthCare Partner to Revolutionize Patient Care with AI-Driven Imaging Solution; Saluda Medical Closes $100M Financing to Expand Neuromodulation Portfolio
Johnson & Johnson MedTech Announced CE Mark Approval for Dual Energy THERMOCOOL SMARTTOUCHTM SF Catheter, Bolstering Capabilities in Cardiac Arrhythmias Treatment On January 10, 2025, Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, announced the receipt of European CE ma...
Read More...
Jan 09, 2025
Roche Secures FDA Clearance for High-Volume Slide Scanner; Pacira Gets 510(k) Approval for Iovera° SmartTip for Chronic Back Pain; ClearCut Presents Clearcoast™ Study Results at San Antonio Breast Cancer Symposium; NanoVibronix Completes UroShield® Pilot Study at University of Michigan; Hologic Finalizes Acquisition of Gynesonics; Movano Health Launches EvieAI Virtual Wellness Assistant.
Roche's Momentum in Digital Pathology Continued With FDA Clearance on its High-Volume Slide Scanner On January 09, 2025, Roche announced that its whole slide imaging system, Roche Digital Pathology Dx, received an additional 510(k) clearance from the United States Food and Drug Administration (FDA). This c...
Read More...
Nov 14, 2024
FDA Grants Approval to Caris Life Sciences for MI Cancer Seek; Johnson & Johnson MedTech Secures FDA IDE Approval for OTTAVA; Organogenesis Shares Positive Interim Data from Second Phase 3 Study of ReNu; LivaNova’s OSPREY Trial Achieves Key Safety and Efficacy Milestones; Hyperfine Launches Advanced Portable MR Brain Imaging Swoop® System Across Europe; Nihon Kohden Strengthens Neurological Solutions Through Ad-Tech Medical Instrument Corporation Acquisition
Caris Life Sciences Received FDA Approval for MI Cancer Seek™ as a Companion Diagnostic (CDx) Test On November 6, 2024, Caris Life Sciences, a leading next-generation AI TechBio company and precision medicine pioneer, announced that the U.S. Food and Drug Administration (FDA) approved MI Cancer Seek™ for u...
Read More...
Sep 26, 2024
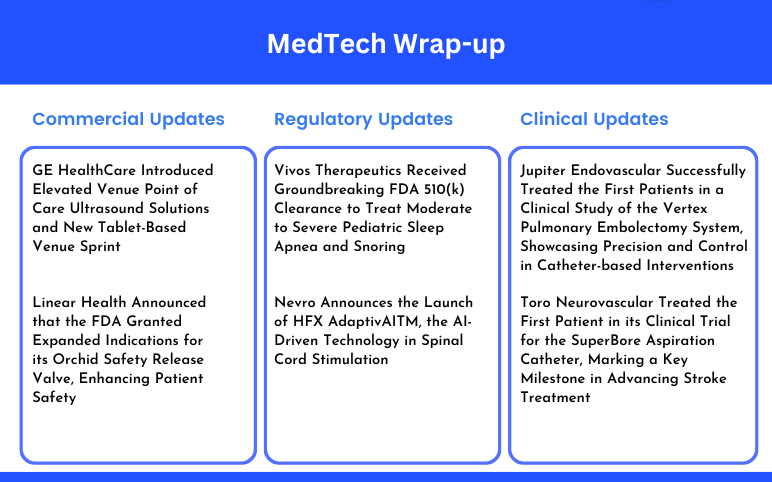
Vivos Therapeutics Receives FDA 510(k) Clearance for Pediatric Sleep Apnea; Nevro Launches AI-Driven Spinal Cord Stimulation; Jupiter Endovascular Treated First Patients in Pulmonary Embolectomy System Study; Toro Neurovascular Advances Stroke Treatment with SuperBore Aspiration Catheter; GE HealthCare Introduces Venue Ultrasound Solutions; Linear Health Expands Orchid Safety Valve Indications
Vivos Therapeutics Received Groundbreaking FDA 510(k) Clearance to Treat Moderate to Severe Pediatric Sleep Apnea and Snoring On September 18, 2024, Vivos Therapeutics, Inc., a premier medical device and technology firm focused on innovative treatments for sleep-related breathing disorders (SRBDs), including obs...
Read More...
May 02, 2024
Xtant Medical’s SimipliGraft TM and SimpliMaxTM Launch; Perelel’s Fertility Support Products Expansion; Medtronic’s InceptivTM FDA Approval; GE Healthcare’s Vital Signs Monitor FDA 510(K) Clearance; Merck’s Pneumonia Vaccine Positive Results; Artivion’s Aortic Devices AATS Win
Xtant Medical Announced the Launch of SimipliGraft TM and SimpliMaxTM for Chronic and Acute Wounds On April 30, 2024, Xtant Medical Holdings, Inc., a global medical technology firm specializing in surgical remedies for spinal disorders, announced the complete commercial availability of two amniotic membran...
Read More...
May 18, 2023
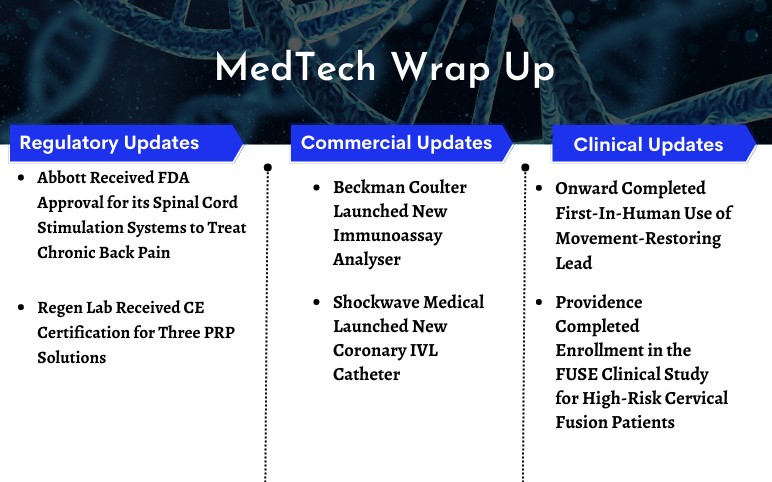
FDA Approves Abbott’s Spinal Cord Stimulation Systems; Regen Lab Received CE Certification for Three PRP Solutions; Beckman Coulter Launched New Immunoassay Analyser; Shockwave Medical’s Coronary IVL Catheter; Providence Completed Enrollment in the FUSE Clinical Study; Onward Completed First-In-Human Use of Movement-Restoring Lead
Providence Medical Technology Completed Enrolment in the FUSE Clinical Study for High-Risk Cervical Fusion Patients On May 11, 2023, Providence Medical Technology, Inc. (PMT), a leader in medical device innovation for spine surgery, closed the enrolment in its FUSE Study which is a prospective, multicenter...
Read More...
Feb 02, 2023
Alma Announced the Launch of Alma Duo; Phillips-Medisize Launched a Pen Injector Platform; FDA Approved Abbott’s Spinal Cord Stimulation; Axonics Received FDA Approval for Rechargeable Sacral Neuromodulation; SELUTION SLR Coronary Sirolimus DEB Study Enrolls First Patient; AtriCure LeAAPS Clinical Trial
Alma announced the Launch of Alma Duo- an Advanced Solution for Sexual Wellness On January 26, 2023, Alma, the core subsidiary of Sisram Medical and one of the leaders of energy-based medical and aesthetics solutions, launched its new sexual wellness product, Alma Duo. Alma Duo is a cutting-edge, safe, non-in...
Read More...
Aug 25, 2022
Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to Acquire enCAGE Coil Precision Ablation System; Boston Heart Diagnostics Launches LipidSeqTM; Rocket VR Health Partners with Penn Medicine’s Abramson Cancer Center; Thermedical’s Clinical Trial to Treat Ventricular Tachycardia
Edwards Pascal Precision Transcatheter Mitral And Tricuspid Valve Repair System Receives CE Mark On August 17, 2022, Edwards Lifesciences Corporation, an American medical technology company headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company announc...
Read More...








-Agonist.png)

