TIGIT
May 17, 2024
GSK and iTeos Alliance Boosts Belrestotug Prospects in TIGIT Space
iTeos shares jump by 40%. GSK and iTeos collaboration for Belrestotug looks like a worthy wait. Hopes to ward off the negative sentiments around the volatile TIGIT space? In the ever-evolving landscape of cancer immunotherapy, the pursuit of effective treatment strategies continues to drive innovation and invest...
Read More...
Jan 04, 2022
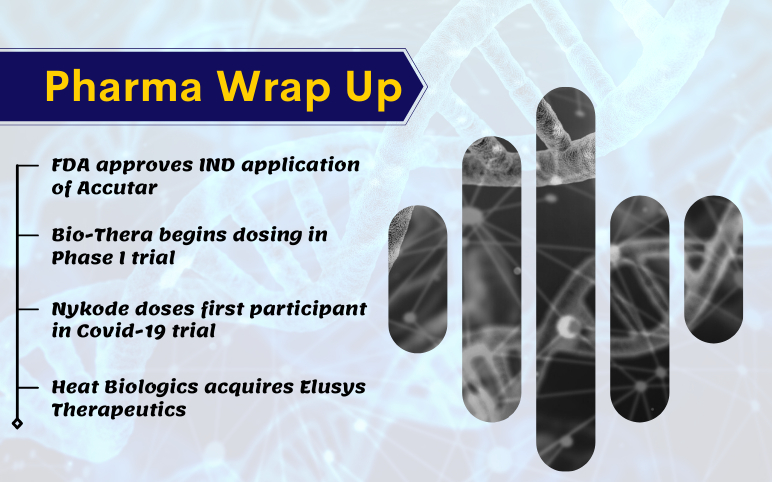
Accutar’s Phase I clinical trial for AC0176; Bio-Thera’s cancer drug, BAT6005; Nykode’s Covid-19 vaccine trial; Heat Biologics acquires Elusys
FDA approves IND application of Accutar for a prostate cancer treatment trial The US Food and Drug Administration (FDA) has granted Accutar Biotechnology’s Investigational New Drug (IND) application for Phase I clinical trial of AC0176 for treating metastatic castration-resistant prostate cancer (mCRPC). An i...
Read More...
Dec 21, 2021
Edwards’ Sapien 3 with Alterra Prestent; Koios Medical’s breast, thyroid cancer-spotting AI; Lineage Collaborates with Genentech; Novartis, BeiGene ink deal
Edwards secures FDA approval for Sapien 3 with Alterra Prestent for Transcatheter Pulmonic Valve Replacement Edwards Lifesciences declared to receive approval from the U.S. Food and Drug Administration (FDA) to use the Edwards SAPIEN 3 transcatheter valve with the Alterra adaptive prestent (SAPIEN 3 with Alterra...
Read More...

-Agonist.png)

