Tissue Heart Valves
Jun 19, 2025
Presidio Medical™ Gains IDE Nod for ULF™ Study, Names Dimas Jiménez CFO; Creo Medical Gains FDA Nod for SpydrBlade™ Flex, Paving Way for U.S. Launch; Penumbra Completes Patient Enrollment in Pivotal STORM-PE RCT; Adona Medical Completes Enrollment in First-in-Human Trial of Novel Interatrial Shunt for Heart Failure; Johnson & Johnson Launches TECNIS Odyssey Intraocular Lens in Europe, Middle East, and Canada for Enhanced Cataract Care; Philips Sets New Benchmark in POCUS Technology with Flash 5100 Launch
Presidio Medical™ Received IDE Approval for Ultra Low Frequency (ULF™) Neuromodulation Clinical Study and Appoints Dimas Jiménez as Chief Financial Officer On June 12, 2025, San Mateo, California–Presidio Medical, Inc., a global clinical-stage medical device company, received Investigational New Drug (IDE)...
Read More...
Jun 18, 2025
Exploring Life-Saving Heart Devices: Innovations Transforming Cardiac Care
Heart disease continues to be a major global health concern, impacting countless lives and placing a significant burden on healthcare systems worldwide. According to the World Health Organization, cardiovascular diseases are responsible for approximately 17.9 million deaths each year, accounting for nearly one-thir...
Read More...
Feb 01, 2024
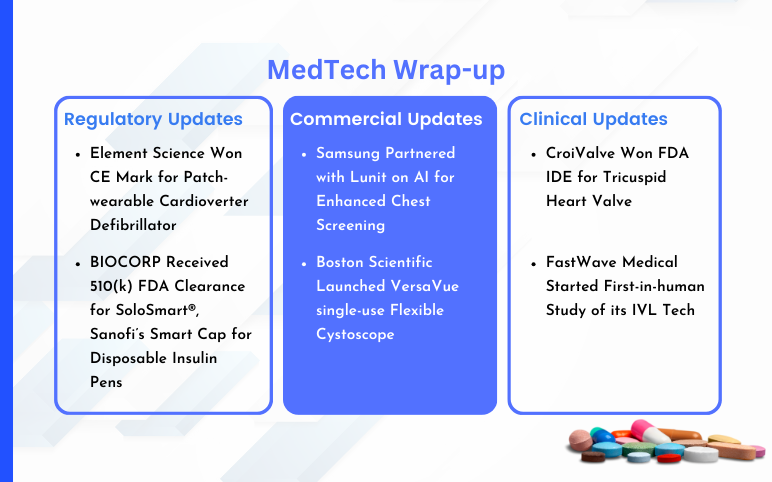
Samsung Partnered with Lunit; Boston Scientific Launched VersaVue single-use Flexible Cystoscope; Element Science’s Patch-wearable Cardioverter Defibrillator; BIOCORP Received 510(k) FDA Clearance for SoloSmart; FDA IDE for CroiValve’s Tricuspid Heart Valve; FastWave Medical’s First-in-human Study of its IVL Tech
Samsung partnered with Lunit on AI for Enhanced Chest Screening On January 25, 2024, Samsung entered into a supply collaboration with Lunit, in order to employ its AI-powered technology for conducting chest screenings. The agreement was signed by Boston Imaging, which serves as the U.S. hub for Samsung's d...
Read More...
Jun 16, 2022
Aurora Spine’s Interbody Fusion Device; LENSAR’s ALLY Adaptive Cataract Treatment System; Abbott’s Mitral & Tricuspid Heart Valve Devices; Boston Scientific’s Acurate Neo2 Aortic Valve System Study; Resolution Medical Acquired Lifetec Group; Paragon 28’s Monkey Rings External Fixation System
US FDA Grants 510K Clearance for Aurora Spine’s Interbody Fusion Device On June 07, 2022, Aurora Spine, Inc. a designer and manufacturer of innovative medical devices, is an emerging growth company focused on delivering new solutions to the spinal implant and pain management industries through a series of screwl...
Read More...



-Agonist.png)

