TRIPLE NEGATIVE BREAST CANCER
May 06, 2025
Abeona Secures FDA Nod for ZEVASKYN, the First Gene Therapy for RDEB; AbbVie Gains FDA Approval for RINVOQ in Giant Cell Arteritis; Lantern Pharma Advances LP-184 with IND Clearance for TNBC Trial; Ichnos Glenmark Earns FDA Fast Track for ISB 2001 in Multiple Myeloma; Rezolute Wins Breakthrough Therapy Designation for Ersodetug in Tumor-Induced Hypoglycemia
FDA Approves Abeona’s ZEVASKYN as First Cell-Based Gene Therapy for RDEB The FDA approved Abeona Therapeutics’ ZEVASKYN (prademagene zamikeracel, or pz-cel), the first and only autologous cell-based gene therapy for treating wounds in patients with recessive dystrophic epidermolysis bullosa (RDEB). ZEVASKYN is d...
Read More...
Apr 22, 2025
FDA Approves Sanofi/Regeneron’s DUPIXENT as First New CSU Therapy in Over a Decade; Gilead’s TRODELVY + KEYTRUDA Shows PFS Benefit in PD-L1+ TNBC; Tempest’s TPST-1495 Gets FDA Orphan Tag for FAP; uniQure’s AMT-130 Granted FDA Breakthrough for Huntington’s; NeuroNOS’ BA-102 Secures FDA Orphan Status for Phelan-McDermid Syndrome
FDA Approves Sanofi/Regeneron’s DUPIXENT as First New Targeted Therapy in Over a Decade for Chronic Spontaneous Urticaria Regeneron Pharmaceuticals and Sanofi have announced that the FDA has approved DUPIXENT (dupilumab) for the treatment of adults and adolescents aged 12 years and older with chronic spontaneous...
Read More...
Dec 09, 2024
How CDK4/6 Inhibitors Are Changing the Cancer Treatment Paradigm?
In the rapidly evolving landscape of cancer therapy, one class of drugs is making waves for its game-changing impact: CDK4/6 inhibitors. These revolutionary agents are rewriting the narrative for treating cancers driven by uncontrolled cell division, particularly hormone receptor-positive, HER2-negative breast canc...
Read More...
Aug 08, 2024
Key Facts to Know About Triple-Negative Breast Cancer in Breast Cancer Awareness Month
October, a month awash in pink, stands as a global testament to Breast Cancer Awareness Month. Pink ribbons flutter in the breeze, pink tee-shirts fill the streets, and pink banners drape buildings. This vibrant display of pink is more than a color; it’s a unified call to action, dedicating a month to raising aware...
Read More...
Jun 25, 2024
AstraZeneca’s AKT Inhibitor TRUQAP Falls Short in Triple Negative Breast Cancer
Triple-negative breast cancer (TNBC) constitutes about 10-15% of all breast cancer cases with nearly 44,000 incident cases in the United States in 2023, as per Delveinsight’s estimates. It is a rare but aggressive form of breast cancer. Treatment for TNBC is more limited than for other breast cancer types. C...
Read More...
Jul 28, 2020
AZ, Daiichi inks an oncology deal; Moderna begins Phase III trial of its COVID-19 vaccine; No good news for Solid’s DMD gene therapy trial
AZ, Daiichi put their trust into TROP2-based drugs, inks an oncology deal to together develop and commercialize DS-1062 AstraZeneca has inked an oncology deal with Daiichi Sankyo to jointly develop and commercialize an antibody-drug conjugate DS-1062 (trastuzumab deruxtecan), worldwide except in Japan. Daiichi ...
Read More...
Oct 16, 2019
New Therapeutic advances that have shifted the Breast cancer market scenario
HR-positive/HER2-negative breast cancer treatment landscape focuses on the complexity of the specific cancer type and the treatment differ according to the biomarker status. The trend suggests a greater bend towards personalized medicine based upon patient biomarkers and physiologic characteristics In our last a...
Read More...
Aug 27, 2019
Zogenix acquires Modis Therapeutics; Amgen buys Otezla; CRISPR slows down the Breast cancer rate
Zogenix pays USD 250M upfront Modis clinical drug Zogenix, a pharmaceutical company focused on advancing rare disease therapies, has collaborated with the Modis therapeutics. Modis Therapeutics is a company aiming to advance in mitochondrial biology to develop disease-modifying therapies for rare genetic diseas...
Read More...
Nov 08, 2018
Gordon and Betty Moore Foundation gives $85M; Omeicos raises; Sanofi signs deal; Lilly grabs
Gordon and Betty Moore Foundation gives USD 85 Million to stop delayed, erroneous diagnostics The Gordon and Betty Moore Foundation is putting up money for improving diagnostics. The Foundation focuses on a plethora of areas, including those outside of healthcare, such as environmental conservation. It has an annua...
Read More...
Oct 22, 2018
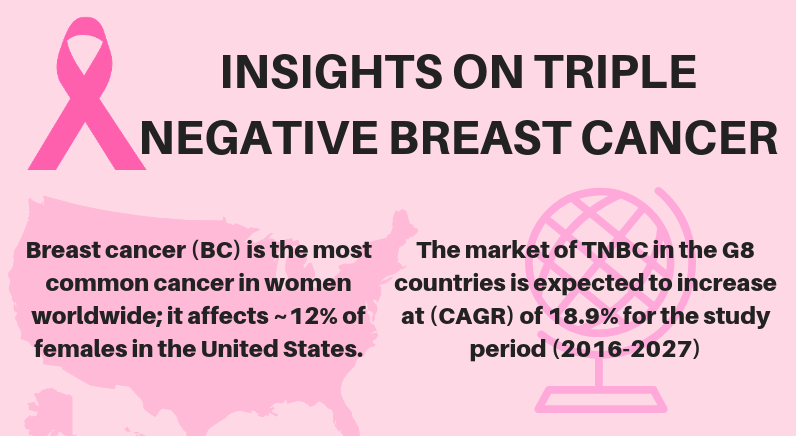
INSIGHTS ON TRIPLE NEGATIVE BREAST CANCER
[caption id="attachment_3465" align="alignnone" width="800"] INSIGHTS ON TRIPLE NEGATIVE BREAST CANCER[/caption] To know more about TRIPLE NEGATIVE BREAST CANCER request for sample pages: https://www.delveinsight.com/dev-sample.php?form_name=Triple-Negative-Breast-Cancer-(TNBC)---Market-Insight,-Epidemi...
Read More...





-Agonist.png)

