X-Ray Devices
May 22, 2025
FDA Clears Fujirebio’s Lumipulse® G Plasma Test for Detecting Alzheimer’s-Linked Amyloid Pathology; Stryker Gains FDA Clearance for OptaBlate® BVN System to Treat Chronic Low Back Pain; Abbott Unveils Clinical Evidence Linking Libre® CGM to Reduced Cardiovascular Events in Diabetes; GE HealthCare Launches AI-Based CleaRecon DL for Enhanced 3D Reconstruction in Interventional Suites; Philips Advances Cardiac Care with Launch of RADIQAL Clinical Study and Real-Time 3D Intracardiac Imaging Catheter in Europe
Fujirebio Received FDA Clearance for Innovative Lumipulse® G Plasma Biomarker Test, Marking Major Advancement in Identifying Amyloid Pathology Linked to Alzheimer’s Disease On May 16, 2025, Fujirebio announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for its Lumipulse® G pT...
Read More...
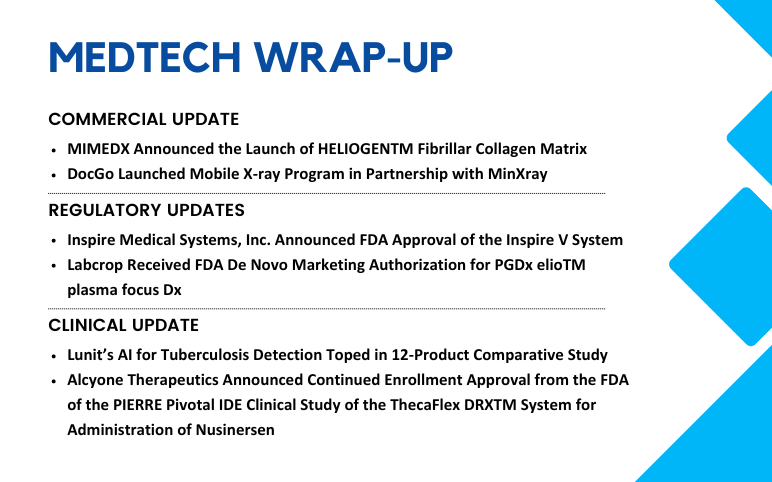
Aug 08, 2024
MIMEDX Launches HELIOGENTM Fibrillar Collagen Matrix; DocGo Launches Mobile X-ray Program; Labcrop’s FDA De Novo Marketing Authorization; Inspire Medical Systems Receives FDA Approval; Alcyone Therapeutics Announces Continued Enrollment Approval from the FDA; Lunit’s AI for Tuberculosis Detection
MIMEDX Announced the Launch of HELIOGENTM Fibrillar Collagen Matrix On July 31, 2024, MiMedx Group, Inc. launched HELIOGEN™ Fibrillar Collagen Matrix, a particulate xenograft product designed to treat complex wounds, especially in surgical environments. HELIOGEN is a shelf-stable product that contains Type...
Read More...
Aug 11, 2022
Rapid Medical’s TIGERTRIEVER 13; Glaukos’s Istent Infinite System; GE Healthcare’s Definium 656 HD; NeuroOne’s Signed Exclusive Development & Distribution Agreement with Zimmer; Kaia Health’s Rise-uP Randomized Controlled Trial; Bluejay’s Symphony IL-6 Test
Rapid Medical Obtains FDA Clearance for the World's Smallest and Only Adjustable Thrombectomy Device On July 26, 2022, Rapid Medical, a leading developer of advanced neurovascular devices, received Food and Drug Administration (FDA) 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions.&n...
Read More...


-Agonist.png)

