Acute-On-Chronic Liver Failure (ACLF) Market Summary
- According to DelveInsight’s analysis, the ACLF market is expected to grow at a CAGR of approximately 11% during the forecast period (2020-2034).
- The ACLF market is set for steady growth, with a robust compound annual growth rate (CAGR) anticipated from 2024 to 2034. This expansion in the 7MM is driven by the introduction of new therapies such as Albumin 5% (PE-A 5%) and VS-01, among others, along with the rising cases of chronic liver diseases, such as hepatitis and non-alcoholic fatty liver disease.
Acute-on-Chronic Liver Failure (ACLF) Market and Epidemiology Analysis
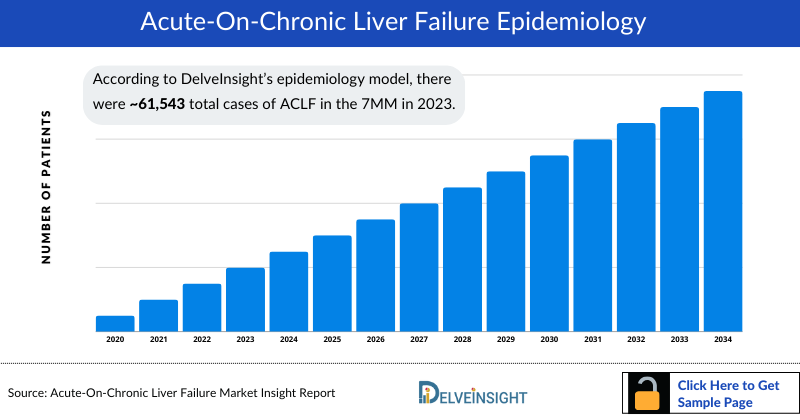
- According to DelveInsight’s estimates, in 2023, there were approximately 61,543 total cases of acute-on-chronic liver failure (ACLF) in the 7MM. Of these, the United States accounted for 21% of the cases, while EU4 and the UK represented 73% and Japan represented 6% of the cases, respectively.
- Currently, there is no approved therapy for ACLF, creating a significant opportunity for pharmaceutical companies. Various ACLF companies, such as Grifols Therapeutics, GENFIT, and Martin Pharmaceuticals, among others, are actively developing their assets to address the limitations of existing management approaches.
- While liver transplantation is effective, challenges such as limited physician awareness, high development costs, and complex administration hinder the market access of new cell-based therapies, potentially stalling advancements in treatment options.
DelveInsight’s “Acute-on-Chronic Liver Failure (ACLF) Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of ACLF, historical and forecasted epidemiology, as well as the ACLF market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The ACLF market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM ACLF market size from 2020 to 2034. The Acute-on-chronic liver failure market report also covers ACLF treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the Acute-on-chronic liver failure market’s potential.
Scope of Acute-on-Chronic Liver Failure Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
ACLF Epidemiology |
|
|
ACLF Treatment Market |
|
|
Acute-on-chronic liver failure Market Analysis |
|
|
ACLF Companies |
|
|
Future opportunity |
The absence of an approved therapy for ACLF presents a significant opportunity for pharmaceutical companies to innovate and develop treatments that address the limitations of current management strategies. As the patient population has been increasing in recent years, this trend is expected to continue, expanding the market size and creating a favorable environment for the introduction of new therapies. The growing need for effective treatments underscores the potential for pharmaceutical players to make substantial advancements in managing ACLF and improving patient outcomes. |
Key Factors Driving the Growth of the Acute on Chronic Liver Failure Market
Rising Chronic Liver Disease Prevalence
The increasing prevalence of chronic liver diseases such as cirrhosis, alcoholic liver disease, hepatitis B and hepatitis C, and MAFLD is leading to a larger pool of patients at risk for ACLF. This rise significantly contributes to the growing incidence of ACLF globally, which is a serious complication with high short-term mortality.
Advances in Medical Treatments
Recent progress in medical treatments, spanning innovative diagnostic tools, targeted drug therapies, and novel combination approaches, has led to improved outcomes for patients with ACLF. These advances include the introduction of new drug classes such as antivirals and immunosuppressants, along with ongoing developments in gene and cell-based therapies that aim to address unmet needs beyond traditional supportive care and organ transplantation.
Rising ACLF Clinical Trial Activities
The ACLF drug development landscape remains limited, with only a handful of companies actively advancing therapies. Genfit leads the field with four assets in its R&D pipeline, including programs currently in Phase 2. Its candidates VS-01, G1090N (a reformulation of NTZ), SRT-015, CLM-022, and VS-02-HE target distinct mechanisms and utilize complementary biological pathways. Beyond these, other notable ACLF candidates in clinical trials include YAQ005 (Yaqrit) and ALBUTEINE (albumin 5%) from Grifols Therapeutics, among others.
Acute-on-Chronic Liver Failure Treatment Market
Acute-on-chronic liver failure overview
ACLF is a syndrome that occurs in patients with chronic liver disease, with or without previously diagnosed cirrhosis and is characterized by acute hepatic decompensation leading to liver failure (manifested by jaundice and an increased international normalized ratio), along with one or more extrahepatic organ failures. This condition is associated with a heightened risk of mortality within 28 days to 3 months from its onset. In some patients, chronic liver disease naturally progresses to cirrhosis, which has two stages: compensated and decompensated. Compensated cirrhosis is characterized by the absence of complications, whereas decompensated cirrhosis is marked by the onset of symptoms such as jaundice, ascites, variceal bleeding, or hepatic encephalopathy. Factors such as viral infections, drug toxicity, alcohol use, ischemic hepatitis, surgery, or sepsis can worsen chronic liver disease, whether or not cirrhosis is present, potentially leading to hepatic and extrahepatic organ failure.
ACLF is further classified into three types based on the underlying liver condition: Type A (underlying non-cirrhotic chronic liver disease), Type B (previously compensated cirrhosis), and Type C (previously decompensated cirrhosis). Thus, ACLF represents a late stage in the progression of chronic liver disease, characterized by both hepatic and extrahepatic organ failure.
Acute-on-chronic liver failure diagnosis
ACLF can be diagnosed in patients presenting with features of acute hepatic decompensation. Common laboratory findings include a prolonged international normalized ratio (INR) of 1.5 or greater, elevated bilirubin and aminotransferase levels, thrombocytopenia with anemia, hypoglycemia, elevated ammonia, signs of acute renal injury (elevated serum creatinine), and abnormal electrolyte levels, such as hypokalemia and hypophosphatemia.
Imaging studies may be necessary to support clinical examination findings and to identify infections, organ involvement, or organ failure. Imaging of the brain, chest, abdomen, and pelvis is considered. Abdominal imaging is particularly important for assessing features of portal hypertension, hepatocellular carcinoma, vascular thrombosis, lymphadenopathy, and spleen size. An abdominal sonogram with Doppler may be useful in patients with concurrent renal injury or vascular thrombosis. Brain imaging (CT or MRI) can help rule out organic causes of altered mental status, while chest imaging is important for excluding pulmonary edema or pneumonia.
Further details related to country-based variations are provided in the report…
Acute-on-chronic liver failure treatment
The management of ACLF involves preventing the precipitating factors that lead to acute hepatic decompensation, providing supportive care, and promptly initiating specific therapies. It also includes preventing and managing complications, assessing the patient's prognosis, and evaluating the need for liver support, including potential liver transplantation. All patients should ideally be hospitalized in a center equipped with the necessary facilities and expertise for liver transplantation.
Acute-on-Chronic Liver Failure Epidemiology
As the ACLF market is derived using a patient-based model, the ACLF epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total cases of ACLF, total cases of ACLF by grades, organ failures associated with ACLF and potential precipitating events of ACLF in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key findings of the Acute-on-Chronic Liver Failure Epidemiology Report
- According to DelveInsight’s epidemiology model, there were approximately 61,543 total cases of ACLF in the 7MM in 2023. These cases are expected to increase during the forecast period (2024-2034), due to the increasing prevalence of chronic liver diseases.
- In 2023, the US accounted for approximately 13,175 total cases of ACLF which is expected to increase by 2034.
- Among the EU4 and the UK, Germany accounted for approximately 16,896 total cases of ACLF in 2023, followed by the UK with nearly 12,321 cases. On the other hand, Italy accounted for the least with approximately 1,728 cases.
- Japan accounted for approximately 3,654 total cases of ACLF in 2023 which is expected to change by 2034.
- Among the total cases of ACLF by grade, there were approximately 6,379 cases for Grade 1, 5,039 cases for Grade 2, and nearly 1,757 cases for Grade 3 in the US in 2023.
- In 2023, among the organ failures associated with ACLF in EU4 and the UK, there were approximately 20,875 cases of liver failure, 17,542 cases of kidney failure, 10,164 cases of cerebral failure, 11,076 cases of coagulation, 6,483 cases of circulation, and 3,268 cases of lungs failure.
- Among the potential precipitating events of ACLF in Japan, there were nearly 529 cases of bacterial infection, 312 cases of gastrointestinal hemorrhage, and 1,096 cases of alcoholism one year prior to the cirrhosis index date, 1,644 cases of infection or GIB or alcohol and 72 other cases in 2023. These cases are expected to change during the forecast period.
Acute-on-Chronic Liver Failure Drug Analysis
Emerging ACLF Drugs
Albumin 5% (PE-A 5%): Grifols Therapeutics
Albumin 5% (PE-A 5%) is an investigational therapy currently being evaluated in a Phase III international study, the APACHE trial. This study focuses on the use of therapeutic plasma exchange (TPE) with albumin replacement to eliminate both endogenous and exogenous toxic substances in patients suffering from ACLF, to prolong patient survival. Concurrently, Grifols is conducting translational research to complement this trial, offering crucial insights into the mechanisms underlying systemic inflammation, organ failure, and ACLF in cirrhosis.
VS-01: GENFIT (Versantis)
VS-01-ACLF is an innovative investigational drug utilizing a proprietary scavenging liposomal technology. It is administered directly into the peritoneal (abdominal) cavity following the drainage of ascites, a common complication in patients with ACLF. VS-01 has received Orphan Drug Designation (ODD) for ACLF from the US FDA.
VS-01 is currently being evaluated in the international UNVEIL-IT Phase II proof-of-concept study. This study aims to assess the efficacy, safety, and tolerability of VS-01 in addition to standard of care (SOC) compared to SOC alone in adult patients with ACLF grades 1 and 2 and ascites.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
Albumin 5% (PE-A 5%) |
Albumin replacement |
Intravenous |
Grifols Therapeutics |
|
III |
|
VS-01 |
Ammonia scavengers |
Intraperitoneal |
GENFIT (Versantis) |
|
II |
|
LIVANTRA (trimetazidine) |
beta-oxidation of free fatty acids inhibitor |
Oral |
Martin Pharmaceuticals |
|
I |
|
XX |
XX |
XXX |
XX |
|
X |
ACLF Drug Class Insights
ACLF Market Outlook
Acute-on-Chronic Liver Failure Drugs Uptake
Acute-on-Chronic Liver Failure Clinical Trial Activities
Acute-on-Chronic Liver Failure Pipeline Development activities
KOL Views on Acute-on-chronic liver failure
Physician’s View
Qualitative Analysis
ACLF Market Access and Reimbursement
Scope of the Acute On Chronic Liver Failure Market Report
- The Acute On Chronic Liver Failure treatment market report covers a segment of key events, an executive summary, and a descriptive overview of ACLF, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the ACLF drugs market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The ACLF treatment market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM ACLF therapeutics market.
Acute-on-chronic liver failure market report insights
- Acute On Chronic Liver Failure Patient Population
- Acute On Chronic Liver Failure Therapeutic Approaches
- ACLF Pipeline Analysis
- ACLF Market Size
- Acute On Chronic Liver Failure Market Trends
- Existing and Future ACLF Market Opportunity
Acute-on-chronic liver failure market report key strengths
- 11 years Forecast
- The 7MM Coverage
- ACLF Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Acute On Chronic Liver Failure Drugs Uptake
- Key Acute On Chronic Liver Failure Market Forecast Assumptions
Acute-on-chronic liver failure Market report assessment
- Current Treatment Practices
- Acute On Chronic Liver Failure Unmet Needs
- Acute On Chronic Liver Failure Pipeline Product Profiles
- ACLF Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions answered in Acute-on-Chronic Liver Failure Market
ACLF Market Insights
- What was the total Acute On Chronic Liver Failure market size, the market size of ACLF by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Albumin 5% (PE-A 5%) affect the treatment paradigm of ACLF?
- How will Albumin 5% (PE-A 5%) compete with other upcoming products and marketed therapies?
- Which Acute-on-chronic liver failure drug is going to be the largest contributor by 2034?
- How would future opportunities affect the Acute On Chronic Liver Failure market dynamics and subsequent analysis of the associated trends?
ACLF Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of ACLF? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to ACLF?
- What is the historical and forecasted ACLF patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent ACLF population during the forecast period (2024–2034)?
- What factors are contributing to the growth of ACLF cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of ACLF? What are the current clinical and treatment guidelines for treating ACLF?
- How many Acute-on-chronic liver failure companies are developing therapies for the treatment of ACLF?
- How many emerging Acute-on-chronic liver failure therapies are in the mid-stage and late stage of development for treating ACLF?
- What are the recent novel therapies, targets, Acute-on-chronic liver failure mechanisms of action, and technologies developed to overcome the limitations of existing Acute-on-chronic liver failure therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What is the 7MM historical and forecasted Acute-on-chronic liver failure treatment market?
Reasons to Buy the Acute-on-Chronic Liver Failure Market
- The Acute On Chronic Liver Failure treatment market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the ACLF market.
- Insights on patient burden/ACLF prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing ACLF market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming ACLF companies in the Acute On Chronic Liver Failure market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Acute On Chronic Liver Failure Companies can strengthen their development and launch strategy.
-market.png&w=256&q=75)

-pipeline-report.png&w=256&q=75)


