C-MET Metastatic NSCLC Market
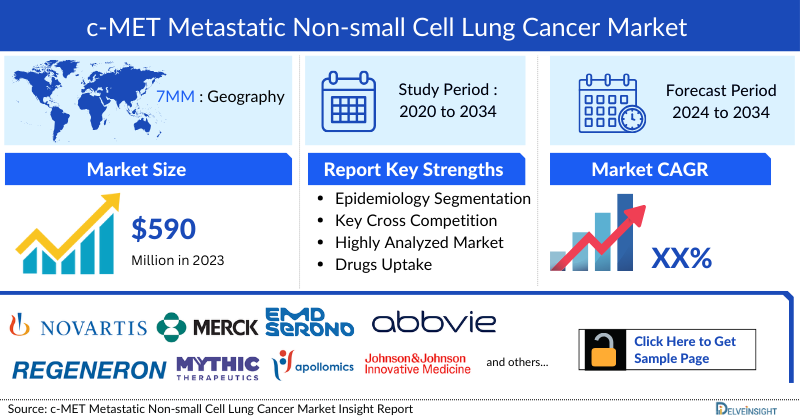
- The total C-MET Metastatic NSCLC Market Size in the 7MM was estimated to be nearly USD 590 million in 2023, which is expected to increase due to the launch of emerging therapies.
- In 2023, the US accounted for the maximum share of the total C-MET Metastatic NSCLC Market in the 7MM, i.e., approximately 75%.
- TABRECTA (capmatinib) and TEPMETKO (tepotinib), two FDA-approved and highly specific small-molecule inhibitors of c-MET exon 14 skipping mutations, are new and important therapeutic options for the treatment of NSCLC patients harboring c-MET alterations. TABRECTA is expected to continue its market-leading position in c-MET NSCLC thanks to its first-to-market advantage.
- In May 2024, preliminary results from the METalmark study revealed that the combination of amivantamab and TABRECTA achieved promising safety and efficacy in advanced NSCLC with MET-driven mutations, with the recommended Phase II dose identified.
- In 2023, off-label use of Crizotinib and other targeted therapies accounted for roughly USD 100 million in the C-MET NSCLC Market in the United States. In comparison, TABRECTA achieved approximately USD 200 million in the same year.
- Novartis has removed TABRECTA from the market in Germany due to "issues" with the AMNOG system. In February, the Federal Joint Committee (G-BA) voted that the added benefit of TABRECTA was "not proven" for treating advanced non-small cell lung cancer with specific genetic mutations. Current stocks of TABRECTA will last until around March 2024, allowing existing patients to continue their treatment. After that, the drug will be available only through named patient schemes, requiring approval for reimbursement by health insurance funds.
- In NSCLC, MET alterations are important therapeutic targets. Despite demonstrated anticancer efficacy, approved MET TKIs, emerging antibody drugs, and antibody-drug conjugates (ADCs) all have room for improvement.
Request for unlocking the sampel page of the C-MET Metastatic NSCLC Treatment Market
Along with comprehensive knowledge of treatment resistance, the best optimal drug sequencing and combinations are urgently needed for better outcomes. Since many NSCLC patients either are unresponsive to current treatment choices or acquire resistance to them, it is critical to keep investigating novel medicines for such people.
DelveInsight’s “c-Mesenchymal-epithelial transition factor (c-MET) Metastatic Non-small Cell Lung Cancer – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of c-MET NSCLC, historical and forecasted epidemiology as well as C-MET Metastatic NSCLC market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The C-MET Metastatic NSCLC Treatment Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM C-MET Metastatic NSCLC market size from 2020 to 2034. The report also covers current C-MET Metastatic NSCLC Treatment Market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
C-MET Metastatic NSCLC Treatment Market: Understanding and Algorithm
NSCLC is the most common type of lung cancer, accounting for approximately 85% of all lung cancers. It is mainly subcategorized into adenocarcinomas, squamous cell carcinomas, large cell carcinomas, and several other types that occur less frequently, including adenosquamous carcinomas and sarcomatoid carcinomas. MET is a proto-oncogene located on chromosome 7q31.2, encoding the c-Met receptor, a transmembrane tyrosine kinase. While c-Met is normally expressed at low levels in healthy epithelial cells, its overexpression—ranging from 2 to 50 times higher than normal—has been observed in various tumor types, including renal cell carcinoma, NSCLC, colorectal cancer, and liver cancer.
C-MET inhibitors have demonstrated anti-tumor effects in non-small cell lung cancer (NSCLC) in both preclinical and clinical studies. However, due to the molecular diversity of NSCLC, it is probable that only a particular subset of patients will experience benefits from c-MET inhibitors.
C-MET Metastatic NSCLC Diagnosis
The diagnosis and staging of c-MET NSCLC are often done at the same time. The tests and procedures used in the diagnosis of NSCLC are Physical Exam and History, Laboratory Tests, Chest x-ray, CT scan (CAT scan), Sputum Cytology, Thoracentesis, Fine-needle aspiration (FNA) biopsy of the Lung, Bronchoscopy and other techniques.
Further details related to diagnosis are provided in the report…
C-MET Metastatic NSCLC Treatment
The results of the standard NSCLC treatment are poor except for the most localized cancers. The newly diagnosed patients with NSCLC are potential candidates for studies evaluating new forms of treatment. There are different types of treatment available for NSCLC; however, mainly 10 types of standard treatment are used, which include Surgery, Radiation therapy, Chemotherapy, Targeted therapy, Immunotherapy, Laser therapy, Photodynamic therapy (PDT), Cryosurgery, Electrocautery, and Watchful waiting. Several c-MET are expected to enter the NSCLC Market; however, given the physicians’ familiarity with the existing products on the market, DelveInsight do not expect new entrants to disrupt the market significantly during the forecast period.
The standard of care for first-line treatment of MET exon 14 skipping in lung cancer that has spread is a targeted therapy MET inhibitor drug called capmatinib or tepotinib. Other lines of treatment may include clinical trials of other MET inhibitors, immunotherapy with or without chemotherapy, or off-label use of a targeted therapy called crizotinib.
C-MET Metastatic NSCLC Epidemiology
The c-MET NSCLC epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total NSCLC Incident Cases, Gender-specific Cases of NSCLC, Age-specific Cases of NSCLC, Total Incident Cases of NSCLC by Histology, Total Incident Cases of NSCLC by Stage, c-MET NSCLC cases, and Line wise treated pool of c-MET NSCLC in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
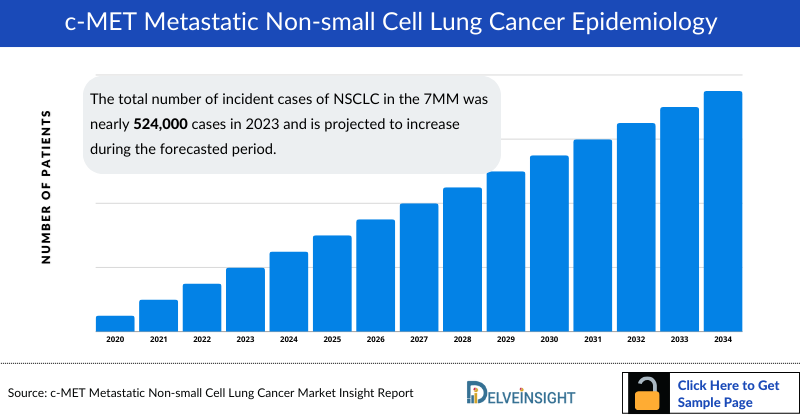
- The total number of incident cases of NSCLC in the 7MM was nearly 524,000 cases in 2023 and is projected to increase during the forecasted period.
- The total number of incident cases of NSCLC in the United States was nearly 202,500 in 2023.
- Although just a small proportion of NSCLC tumors express cMET at high levels, a considerably larger population has tumors that overexpress cMET at lower levels.
- The total number of cases in the United States of c-MET NSCLC was estimated to be nearly 12,000 cases in 2023, which is expected to show positive growth by 2034.
- Tumor biomarker testing rate is around 70% in the US for c-MET NSCLC.
- Among EU4 and the UK, Germany had the highest number of cases of c-MET NSCLC in 2023 and is expected to show positive growth by 2034.
C-MET Metastatic NSCLC Drug Chapters
The drug chapter segment of the C-MET Metastatic NSCLC Therapeutics Market Report encloses a detailed analysis of the marketed and the late-stage (Phase III) C-MET Metastatic NSCLC pipeline drugs analysis. The marketed drugs segment encloses drugs such as TABRECTA (Novartis) and TEPMETKO (EMD Serono/Merck KGaA). The drug chapter also helps understand the c-MET NSCLC clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
C-MET Metastatic NSCLC Marketed Drugs
- TABRECTA (capmatinib): Novartis
TABRECTA (capmatinib) is an oral prescription medicine used to treat adults with NSCLC that has spread to other parts of the body (metastatic) and whose tumors have an abnormal mesenchymal-epithelial transition (MET) gene. The MET protein belongs to a family of enzymes called receptor tyrosine kinases, which are involved in the growth of cells. In NSCLC patients with ‘METex14 skipping,’ an abnormal form of the MET protein is produced that causes cancer cells to divide and grow uncontrolled. Capmatinib is a kinase inhibitor that attaches to this abnormal MET protein inside cancer cells. This stops the effect of MET, helping slow down the growth and spread of the cancer.
In August 2022, the US Food and Drug Administration (FDA) granted TABRECTA regular approval for adult patients with metastatic NSCLC whose tumors have a mutation leading to MET exon 14 skipping, as detected by an FDA-approved test. In May 2020, the US FDA granted TABRECTA accelerated approval for the same indication based on the initial overall response rate and duration of response in the GEOMETRY mono-1 trial.
- TEPMETKO (tepotinib): EMD Serono/Merck KGaA
TEPMETKO (tepotinib) is a kinase inhibitor indicated for treating adult patients with metastatic NSCLC harboring mesenchymal-epithelial transition (MET) exon 14 skipping alterations. It contains the active ingredient tepotinib (as a hydrochloride monohydrate) and is an oral medication. Patients taking TEPMETKO should be warned of the increased risk of severe or fatal interstitial lung disease/pneumonitis, hepatotoxicity, and embryo-fetal toxicity, requiring the use of effective contraception during and shortly after treatment.
In February 2024, the FDA granted full approval to TEPMETKO for adult patients with metastatic NSCLC harboring MET exon 14 skipping alterations. In February 2021, Merck announced that the US FDA had approved TEPMETKO through accelerated.
Note: Detailed list will be provided in the final report.
Note: Detailed marketed and emerging therapies assessment will be provided in the final report.
C-MET Metastatic NSCLC Emerging Drugs
- Telisotuzumab Vedotin: AbbVie
Telisotuzumab vedotin (ABBV-399; Teliso-V) is a first-in-class antibody-drug conjugate (ADC) that uses a cleavable linker to combine a recombinant c-MET-targeting humanized monoclonal antibody (ABT-700) and monomethyl auristatin E (MMAE), a potent inhibitor of microtubule polymerization. The company is evaluating telisotuzumab vedotin in multiple phases to treat patients with NSCLC.
In November 2023, the results of Phase II of LUMINOSITY/M14-239 trial of telisotuzumab vedotin were announced. The trial showed a meaningful response rates and other clinically relevant outcomes in patients with c-Met protein overexpressed, EGFR wild-type, advanced or metastatic non-squamous NSCLC.
According to the company, telisotuzumab vedotin is anticipated to receive accelerated approval for 2L+ NSCLC by 2025, with anticipated submission in 2024. Apart from that, in January 2022, AbbVie announced that the FDA granted Breakthrough Therapy Designation (BTD) to investigational telisotuzumab vedotin for the treatment of patients with advanced/metastatic epidermal growth factor receptor wild-type NSCLC with high levels of c-MET overexpression whose disease has progressed on or after platinum-based therapy.
- REGN5093 and REGN5093-M114: Regeneron Pharmaceuticals
Regeneron is developing both ADC and bispecific antibody for MET NSCLC. The company is conducting Phase I/II for both drugs. REGN5093-M114 is an ADC composed of a novel linker-payload (M114, carrying the maytansine derivative M24, a potent inhibitor of microtubule assembly) covalently bound to lysine residues on a MET-targeting human IgG4p bispecific antibody, REGN5093. In preclinical models of MET overexpressing cancers, REGN5093-M114 demonstrated significant dose-dependent antitumor activity.
Note: Detailed list will be provided in the final report.
Note: Detailed list will be provided in the final report.
C-MET Metastatic NSCLC Market Outlook
Traditionally, when investigators looked at MET as a driver, they primarily looked at gene amplifications. There is a small population of patients who do have an amplification of the MET gene. In addition, there were reports several years ago that drugs effectively targeting MET could potentially be effective approaches in such cases. However, more recently, there has been a recognition that a significant percentage of patients with NSCLC have a mutation. A host of different mutations led to the same consequence, which is the skipping of MET exon 14 in the eventual product of MET.
This leads to a decreased ability for MET to be destroyed, and that has more recently been recognized as a vital driver mutation in approximately 3–4% of patients with NSCLC. Two MET TKIs, TABRECTA and TEPMETKO, are currently approved globally for treating METex14+ NSCLC. The only MET inhibitor approved in China is savolitinib. Subsequent treatment options may include participating in clinical trials testing different MET inhibitors, utilizing immunotherapy with or without chemotherapy, or considering the off-label use of the targeted therapy crizotinib.
The common standard of care in first-line therapy for NSCLC, without oncogenic drivers, involves the use of platinum-based chemotherapy or immune checkpoint inhibitors (ICIs) either as single components or in combination, depending on the level of expression of programmed death-ligand 1 (PD-L1) or potential contraindications such as poor performance status, often found in patients of advanced age. For patients advancing to second-line therapy, monotherapy with ICIs should be considered if not previously given. Patients with oncogenic-driven NSCLC should receive targeted therapy where available, as the efficacy of standard therapies might be impaired in oncogene-addicted disease.
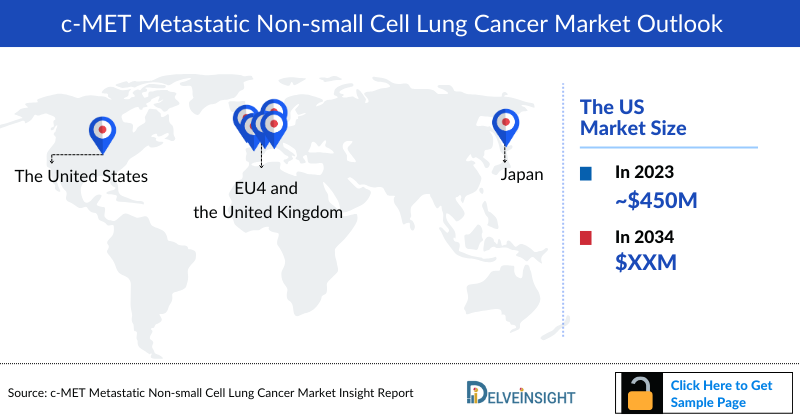
- The total C-MET Metastatic NSCLC Market Size in the US was estimated to be nearly USD 450 million in 2023, which is expected to increase due to the launch of emerging therapies, increasing use of biomarker testing, and increasing incidence of C-MET Metastatic NSCLC.
- Having gained a first-to-market advantage over TEPMETKO, DelveInsight anticipates TABRECTA to maintain its market-leading position among currently marketed therapies in the US that target c-MET in NSCLC.
- C-MET Metastatic NSCLC Companies like AbbVie, Mythic Therapeutics, Regeneron Pharmaceuticals, and others are targeting c-met overexpressed NSCLC patients. Telisotuzumab Vedotin is expected to have the first-mover advantage among all emerging therapies for c-Met overexpressed NSCLC.
- In 2023, Germany captured the highest C-MET Metastatic NSCLC Market Size among the EU4 and the UK.
C-MET Metastatic NSCLC Drugs Uptake
This section focuses on the uptake rate of potential C-MET Metastatic NSCLC drugs expected to be launched in the market during 2020–2034. The C-MET Metastatic NSCLC treatment market landscape has experienced a profound transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing cancer care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Further detailed analysis of emerging therapies drug uptake in the report…
C-MET Metastatic NSCLC Pipeline Development Activities
The C-MET Metastatic NSCLC therapeutics market report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key C-MET Metastatic NSCLC Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The C-MET Metastatic NSCLC therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for c-MET NSCLC emerging therapy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on c-MET NSCLC evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including oncologists, radiation oncologists, surgical oncologists, and others.
DelveInsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as MD, The University of Texas MD Anderson Cancer Center, MD, Duke Cancer Institute at Duke University, PhD, MD, MPH, Dana-Farber Brigham Cancer Center, Michigan State University, Director, Massachusetts General Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or c-MET NSCLC market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
C-MET Metastatic NSCLC Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most crucial primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and this clearly explains the drug's side effects in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
C-MET Metastatic NSCLC Therapeutics Market Access and Reimbursement
The current scenario of therapeutics for NSCLC is mainly based on the use of targeted therapies and immunotherapy. The current paradigm is mainly associated with treatment specific to mutations that occur in NSCLC.
Further detailed analysis of emerging therapies drug uptake in the report…
C-MET Metastatic NSCLC Therapeutics Market Report Scope
- The C-MET Metastatic NSCLC therapeutics market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the C-MET Metastatic NSCLC treatment market, historical and forecasted C-MET Metastatic NSCLC treatment market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The C-MET Metastatic NSCLC therapeutics market report provides an edge while developing business strategies by understanding trends through SWOT analysis, expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM C-MET Metastatic NSCLC drugs market.
C-MET Metastatic NSCLC Therapeutis Market Report Insights
- Patient-based C-MET Metastatic NSCLC Market Forecasting
- Therapeutic Approaches
- c-MET NSCLC Pipeline Drugs Analysis
- c-MET NSCLC Market Size and Trends
- Existing and Future C-MET Metastatic NSCLC Market Opportunity
C-MET Metastatic NSCLC Therapeutics Market Report Key Strengths
- 11 Years C-MET Metastatic NSCLC Market Forecast
- The 7MM Coverage
- c-MET NSCLC Epidemiology Segmentation
- Key Cross Competition
- C-MET Metastatic NSCLC Drugs Uptake
- Key C-MET Metastatic NSCLC Market Forecast Assumptions
C-MET Metastatic NSCLC Therapeutics Market Report Assessment
- Current C-MET Metastatic NSCLC Treatment Market Practices
- C-MET Metastatic NSCLC Unmet Needs
- C-MET Metastatic NSCLC Pipeline Drugs Analysis Profiles
- C-MET Metastatic NSCLC Drugs Market Attractiveness
- Qualitative Analysis (SWOT Analysis and Conjoint Analysis)
FAQs
- What was the C-MET Metastatic NSCLC treatment market size, the C-MET Metastatic NSCLC therapeutics market size by therapies, market share (%) distribution in 2023, and what would it look like by 2034? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of c-MET NSCLC?
- What are the disease risks, burdens, and C-MET Metastatic NSCLC Unmet Needs? What will be the growth opportunities across the 7MM concerning the patient population with c-MET NSCLC?
- Who is the major competitor of TABRECTA in the market?
- What are the current options for the treatment of c-MET NSCLC? What are the current guidelines for treating c-MET NSCLC in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy
- The C-MET Metastatic NSCLC drugs market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the C-MET Metastatic NSCLC drugs market.
- Insights on patient burden/disease C-MET Metastatic NSCLC Prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing C-MET Metastatic NSCLC drugs market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles
- The Next Chapter in NSCLC Treatment Space: Recent Discoveries and Innovations
- Novel mutation-targeting therapies in the horizon to relieve the global healthcare burden NSCLC poses
- Non-Small Cell Lung Cancer Market: Treatments and Market Forecast
- Evaluating Key Advancements and Emerging Therapies in EGFR-Non Small Cell Lung Cancer Treatment Market
- Novel Insights Into The Non-Small Cell Lung Cancer Market
- Evolving Landscape for Rare Biomarkers in Non-Small Cell Lung Cancer
- Latest DelveInsight Blogs
Related Infographics of the Report
-metastatic-non-small-cell-lung-cancer-marke.png&w=256&q=75)