Exosome Therapies Market Summary
- The Exosome Therapies Market Size is anticipated to grow with a significant CAGR during the forecast period (2025-2034).
- Extracellular vesicles serve as vehicles for the transfer of different molecules between cells (proteins, lipids, DNA, RNA, miRNAs, etc.) and are involved in numerous physiological and pathological processes such as the removal of unwanted proteins, antigen presentation, genetic exchange, immune response, angiogenesis, inflammation, tumor metastasis, and dissemination of pathogens or oncogenes.
Exosome Therapies Market and Epidemiology Analysis
- In recent years, engineered exosomes have emerged as a noteworthy class of biogenic nanoparticles. By precisely manipulating the cargo and surface markers of exosomes, engineered exosomes have gained enhanced anti-inflammatory, immunomodulatory, and tissue-reparative abilities, providing new prospects for the treatment of autoimmune diseases.
- Although exosome technology is still very much in its clinical infancy, it represents an innovative drug delivery platform that is starting to make its way into the clinic. At present, extracellular vesicles are being investigated for a range of potential therapeutic and diagnostic uses.
- CAP-1002 (deramiocel) is designed to slow disease progression in patients with Duchenne Muscular Dystrophy by leveraging its immunomodulatory, anti-inflammatory, and anti-fibrotic properties, with the potential to enhance skeletal and cardiac muscle function. If Capricor Therapeutics receives FDA approval to market deramiocel for treating DMD, the company could qualify for a Priority Review Voucher (PRV) under the rare pediatric disease program.
- Exosomes can be exploited for diverse therapeutic applications, including chemotherapy, gene therapy, and photothermal therapy. Moreover, their capacity for homotypic targeting and self-recognition provides opportunities for personalized medicine.
Factors Impacting the Exosome Therapies Market Growth
- Rising cancer burden: Growing number of cancer cases globally driving demand for exosome-based immunotherapy and targeted delivery systems, with substantial spending on cancer screening and treatment
- Increasingly sophisticated manufacturing technologies: Development of scalable and efficient exosome isolation, purification, and characterization methods improving product quality and therapeutic efficacy
- Shift toward personalized therapies: Increasing focus on precision medicine creating demand for patient-specific, targeted treatment options that exosomes uniquely provide
- Minimally invasive treatment preference: Growing demand for therapies with lower side effects compared to conventional treatments, particularly in oncology, neurology, and regenerative medicine
- Liquid biopsy adoption: Growing use of advanced diagnostic tests including exosome-based liquid biopsy kits for non-invasive disease detection and monitoring
- Pharmaceutical and biotech sector investment: Heavy investments from pharmaceutical, biotechnology, and private sector companies accelerating commercialization of novel exosome therapeutics
- Favorable regulatory frameworks: Increasingly supportive regulatory environment facilitating faster approval pathways and clinical development
- Demographic shift: Aging population with enhanced life expectancy leading to higher incidence of age-related chronic diseases amenable to exosome therapy
DelveInsight’s “Exosome Therapies Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the exosome therapies, historical and Competitive Landscape as well as the exosome therapies therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The exosome therapies market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM exosome therapies market size from 2020 to 2034. The report also covers current exosome therapies treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Exosome Therapies Market |
|
|
Exosome Therapiess Market Size | |
|
Exosome Therapies Companies |
|
|
Exosome Therapies Epidemiology Segmentation |
|
Exosome Therapies Treatment Landscape
Exosome Therapies Overview
Exosome therapies are an emerging class of regenerative medicine and targeted drug delivery. These nanoscale extracellular vesicles, naturally secreted by cells, carry bioactive molecules such as proteins, RNAs, and lipids that can influence recipient cells and modulate disease processes. Unlike traditional stem cell therapies, exosome-based approaches offer a cell-free alternative with lower immunogenic risk and the ability to cross biological barriers like the blood-brain barrier. They are being actively explored across multiple therapeutic areas, including neurological disorders (e.g., traumatic brain injury, Parkinson’s Disease), oncology (via tumor-targeted drug delivery or immune modulation), and regenerative medicine (such as wound healing and cardiac repair).
The most studied types of extracellular vesicles are exosomes and microvesicles (MVs), exosomes are nano-sized extracellular particles, naturally produced by all cells, which can be manipulated to incorporate therapies and target cells with high specificity.
Further details related to country-based variations are provided in the report...
Exosome Therapies Epidemiology
The exosome therapies epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total cases of selected indications for exosome therapy, total eligible patient pool for exosome therapy in selected indications and total treated cases in selected indications for exosome therapy in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
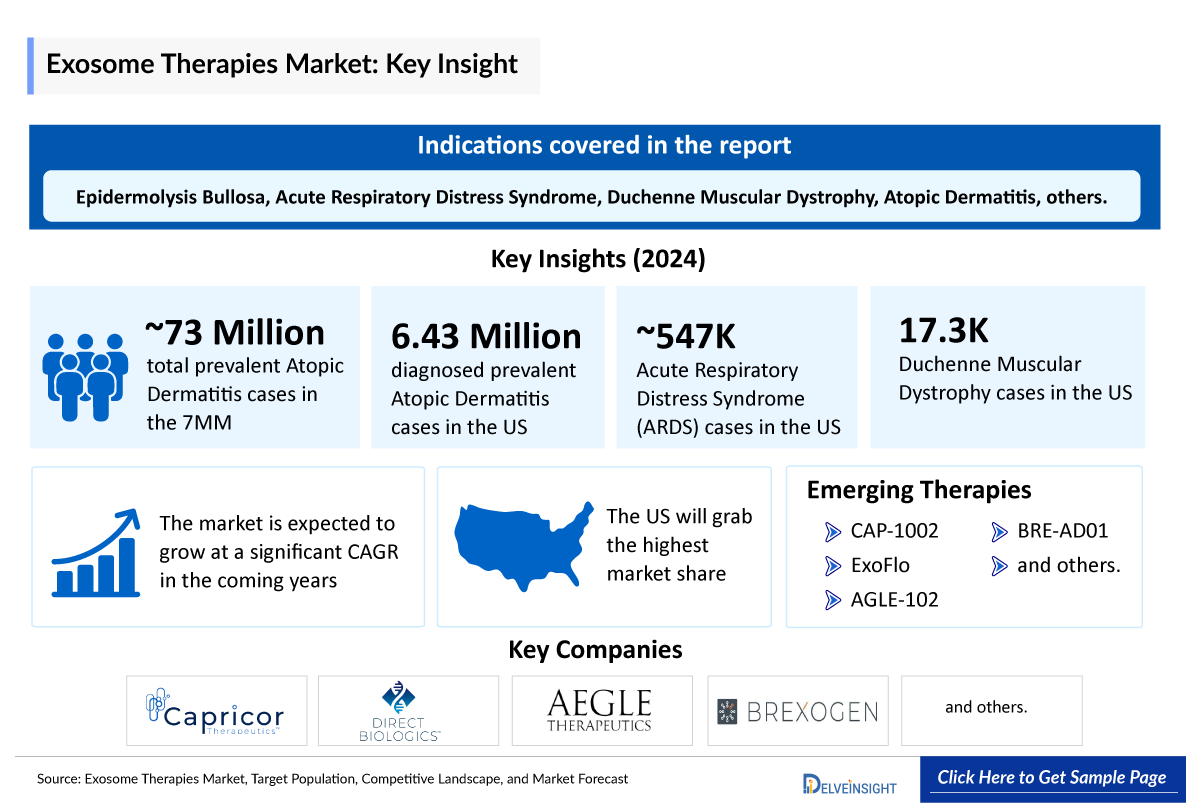
- The total prevalent cases of atopic dermatitis in the 7MM were 72,977,400 in 2024.
- In EU4 and the UK, Germany accounted for the highest number of atopic dermatitis treated cases with approximately 6,428,900 in 2024.
- Among the EU4, Germany accounted for the highest number of Epidermolysis Bullosa prevalent cases, followed by Italy, whereas Spain accounted for the lowest number of cases.
- Japan accounted for 3,300 prevalent Duchenne Muscular Dystrophy cases in 2024.
Exosome Therapies Epidemiology Segmentation
- Total Cases of Selected Indications for Exosome Therapy
- Total Eligible Patient Pool for Exosome Therapy in Selected Indications
- Total Treated Cases in Selected Indications for Exosome Therapy
Recent Developments In Exosome Therapies Treatment Landscape
- In June 2025, Capricor Therapeutics announced positive 4-year safety and efficacy results from the HOPE‑2 open-label extension trial of deramiocel in patients with Duchenne muscular dystrophy. The data showed enduring preservation of cardiac function, including left ventricular ejection fraction and continued slowing of skeletal muscle decline as measured by Performance of the Upper Limb version 2.0 (PUL).
- In June 2025, Rion Aesthetics, a globally recognized leader in regenerative aesthetics, announced that the U.S. Food and Drug Administration (FDA) has approved the initiation of a Phase 1 investigator-initiated clinical trial under an Investigational New Drug (IND) application. The trial will evaluate intradermal injections of its Purified Exosome Product™ (PEP™). This study is independently sponsored and conducted by the Clinical Testing Center of Beverly Hills, with Dr. John H. Joseph, a double board-certified facial plastic surgeon in California, serving as the lead investigator.
- In April 2025, RoosterBio announced a new collaboration with Thermo Fisher Scientific. This collaboration aims to accelerate the availability of new, potentially life-saving cell and exosome therapies that have the potential to revolutionize the treatment of degenerative disease.
- In January 2025, Capricor Therapeutics announced the completion of the submission of its BLA to the US FDA for seeking full approval for deramiocel, an investigational cell therapy, to treat patients diagnosed with DMD cardiomyopathy.
- In January 2025, NurExone Biologic announced that the company had acquired a master cell bank from the US manufacturer for an undisclosed amount.
- In July 2024, Aruna Bio announced the issuance of a US Patent for the composition of matter for neural exosomes used to deliver therapeutics to the brain.
|
S.No. |
Indications |
Estimated Cases in 2024 in the US |
|
1 |
Epidermolysis Bullosa |
~31,200 (Prevalence) |
|
2 |
Acute Respiratory Distress Syndrome |
~547,100 (Incidence) |
|
3 |
Duchenne Muscular Dystrophy |
~17,300 (Prevalence) |
|
4 |
Atopic Dermatitis |
~32,759,500 (Prevalence) |
NOTE: The list of indications is not exhaustive, and will be provided in the final report. Also, numbers are indicative and are subject to change as per report updation...
Exosome Drug Analysis
The drug chapter segment of the exosome therapies reports encloses a detailed analysis of late-stage (Phase III and Phase II) Exosome Therapies pipeline drugs. It also helps understand the exosome therapies clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Exosome Emerging Drugs
CAP-1002: Capricor Therapeutics
CAP-1002 contains cardiosphere-derived cells, a type of heart cell progenitor, or precursor cell, that secretes tiny vesicles called exosomes. These exosomes then modulate the activity of the immune system and stimulate cellular regeneration. Recently, the company has completed the submission of its BLA to the US FDA seeking full approval for deramiocel to treat patients diagnosed with Ducchene Muscular Dystrophy.
- In June 2025, Capricor Therapeutics provided a regulatory update on its CAP-1002 BLA for Duchenne muscular dystrophy-associated cardiomyopathy. The U.S. FDA confirmed no advisory committee meeting is needed and plans an in-person late-cycle review in mid-July, with a PDUFA target date set for August 31, 2025. The company also highlighted four-year HOPE-2 extension data showing sustained preservation of cardiac function.
- The Phase III HOPE-3 trial of deramiocel in DMD is fully enrolled and on track to deliver top-line data from Cohort A in the fourth quarter of 2024. Deramiocel for the treatment of DMD, has received Orphan Drug Designation from the FDA and EMA. The regulatory pathway for deramiocel is supported by RMAT (Regenerative Medicine Advanced Therapy Designation) in the US and the Advanced Therapy Medicinal Product (ATMP) Designation in the European region.
ExoFlo: Direct Biologics
ExoFlo is an extracellular signal product isolated from human bone marrow mesenchymal stem cells (MSCs) that contains growth factors and extracellular vesicles including exosomes. Direct Biologics is currently conducting the global Phase III EXTINGUISH Acute Respiratory Distress Syndrome clinical trial of ExoFlo for the treatment of hospitalized adults with moderate-to-severe ARDS. In addition, the Company has initiated Phase I clinical trials with ExoFlo for the treatment of ulcerative colitis and Crohn’s disease, and expanded access trials in solid abdominal organ transplantation and severe ARDS patients.
Comparison of Key Emerging Exosome Therapies | ||||
|
Product |
Company |
RoA |
Phase |
Indication |
|
CAP-1002 |
Capricor Therapeutics |
IV |
Registration Phase |
Duchenne Muscular Dystrophy |
|
ExoFlo |
Direct Biologics |
IV |
III |
Moderate-to-severe ARDS |
|
Purified Exosome Product (PEP) |
RION |
SC |
II |
Diabetic foot ulcer, Osteoarthritis |
|
AGLE-102 |
Aegle Therapeutics |
Topically |
I/II | |
|
BRE-AD01 |
Brexogen |
SC |
I | |
Note: Detailed emerging therapies assessment will be provided in the final report...
Exosome Therapies Market Outlook
Exosome therapies are a new and exciting area of medicine that could help treat many diseases. Exosomes are tiny particles released by cells that carry important signals, such as proteins and genetic material, to other cells. They help cells communicate and can be used to deliver treatments directly to the parts of the body that need them. Currently, there are no approved exosome-based therapies in the market.
Exosomes are important in both causing and treating disease. For example, in cancer, tumor cells release exosomes that help the cancer grow and spread. But researchers are also using exosomes from healthy cells to slow disease, reduce inflammation, and repair damaged tissue. Because exosomes can travel easily through the body and are less likely to cause side effects, they are seen as a powerful new way to treat difficult diseases. With more research, exosome therapies could become a new option for patients who need better treatments.
What are the differences between CAR-T cells vs. CAR-Exosome Agents? | |||
|
Parameters |
CAR-Exosome |
CAR cells |
Comments |
|
Risk of developing secondary T-cell tumors |
No |
Yes |
The use of genetically engineered CAR cells poses a risk of subsequent tumor growth. |
|
Risk of immune response and cytokine storm |
No |
Yes |
The use of genetically engineered CAR cells is connected with the risk of inducing a host immune response and a potentially fatal cytokine storm. |
|
Ease of manufacturing |
Yes |
No |
Every batch requires the creation of new CAR cells from stem cells, which takes three months. CAR-EVs can be produced on a continual basis. |
|
Immunological memory |
Yes |
Yes |
The immune system has the ability to maintain cancer cell memory. This enables for a swift and broad response in the event of a recurrence. Cell therapy does this by allowing genetically modified cells to survive in the body long after they were initially treated. Exosome therapy does this by giving cancer antigens to the immune system, which then generates lymphocytes that recognize cancer. |
The majority of extracellular vesicles-based therapies derived from mesenchymal stromal stem cells, dendritic cells, and genetically unaltered cells, are being developed or utilized in clinical studies. Owing to their homotypic targeting effects and self-recognition capabilities, exosomes and their hybrid systems are being increasingly explored as alternatives to conventional drug carriers. In addition, multifunctional exosomes can be generated by the simultaneous loading of various therapeutic cargoes. Several exosome-based products, such as ExoFlo for acute respiratory distress syndrome and CAP-1002 for duchenne muscular dystrophy treatment, are in various stages of clinical development. Capricor Therapeutics has entered an exclusive agreement with Nippon Shinyaku for the commercialization and distribution of CAP-1002 for DMD in the US and Japan. Currently, in discussions with the FDA, Capricor Therapeutics aims to position CAP-1002 as a potential best-in-class cell therapy for DMD upon approval.
A few Exosome Companies, including Capricor Therapeutics, Direct Biologics, Aegle Therapeutics, Brexogen, RION, and others are involved in developing drugs for Exosome therapies for various indications such as duchenne muscular dystrophy, moderate-to-severe ARDS recessive dystrophic epidermolysis bullosa, atopic dermatitis and diabetic foot ulcer, osteoarthritis and others. Overall, this is an exciting new class of agents with great potential for development.
Exosome therapies Drugs Uptake
This section focuses on the uptake rate of potential Exosome therapies glues expected to be launched in the market during 2025–2034.
Exosome therapies Clinical Trial Analysis
The Exosome pipeline report provides insights into different Exosome therapies clinical trials within Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
The presence of numerous drugs under different stages is expected to generate immense opportunity for Exosome therapies market growth over the forecasted period.
Exosome Therapies Pipeline Development Activities
The Exosome therapies clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Exosome therapies therapies.
Latest KOL Views on Exosome Therapies
To keep up with current and future market trends, we take Industry Experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on Exosome therapies evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. University such as Harvard Medical School and others.
Their opinion helps understand and validate current and emerging therapy treatment patterns or Exosome therapies market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
What KOLs are saying about the Potential Exosome Therapies Patient Population |
|
“Exosomes are nature’s nanocarriers capable of delivering proteins, RNA, and drugs across the blood-brain barrier. Our research focuses on engineering exosomes for targeted delivery in glioblastoma and neurodegenerative diseases. These vesicles offer unparalleled potential for precision medicine in CNS disorders.”
|
Exosome Therapies Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Exosome Therapies Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Note: List to be continued in the final report…
Scope of the Exosome Therapies Market Report
- The Exosome Therapies Market report covers a segment of key events, an executive summary, and a descriptive overview of the exosome therapies, explaining its mechanism, and emerging exosome therapies.
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current landscape.
- A detailed review of the exosome therapies market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Exosome Therapies Market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM exosome therapies market.
Exosome Therapies Market Report Insights
- Exosome Therapies Targeted Patient Pool
- Exosome Therapies Therapeutic Approaches
- Exosome Therapies Pipeline Analysis
- Exosome Therapies Market Size and Trends
- Existing and future Market Opportunity
Exosome Therapies Market Report Key Strengths
- Ten years Forecast
- The 7MM Coverage
- Key Cross Competition
- Exosome Therapies Drugs Uptake
- Key Exosome Therapies Market Forecast Assumptions
Exosome Therapies Market Report Assessment
- Current Exosome Therapies Treatment Practices
- Exosome Therapies Unmet Needs
- Exosome Therapies Pipeline Product Profiles
- Exosome Therapies Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Exosome Therapies Market Drivers
- Exosome Therapies Market Barriers
Key Questions Answered In The Exosome Therapies Market Report:
- What was the exosome therapies total market size, the market size by therapies, market share (%) distribution, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative market for exosome therapies?
- What are the pricing variations among different geographies for approved therapies?
- What are the risks, burdens, and unmet needs of treatment with exosome therapies? What will be the growth opportunities across the 7MM for the patient population of exosome therapies?
- What are the key factors hampering the growth of the exosome therapies market?
- What are the indications for which exosome therapies are being developed to overcome the limitations of existing treatments?
- What key designations have been granted to exosome therapies?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to buy Exosome Therapies Market Forecast Report
- The Exosome Therapies Market report will help develop business strategies by understanding the latest trends and changing dynamics driving the exosome therapies Market.
- Understand the existing Exosome Therapies market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming Exosome companies in the Exosome Therapies market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the conjoint analysis section to provide visibility around leading indications.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Exosome Therapies Market so that the upcoming players can strengthen their development and launch strategy.