Hepatorenal Syndrome Market Summary
- The Hepatorenal Syndrome market size is forecasted to maintain a consistent Compound Annual Growth Rate (CAGR) between 2024 and 2034. This growth in Hepatorenal Syndrome market revenue is primarily driven by improvements in diagnostic methods, increased awareness of the condition, and a rising incidence of reported cases.
- The current Hepatorenal Syndrome market is marked by less competition as there are only a few companies including Mallinckrodt Pharmaceuticals and others whose assets are approved for the treatment of Hepatorenal Syndrome.
- To drive the Hepatorenal Syndrome market in future years, several Hepatorenal Syndrome companies such as Noorik Biopharmaceuticals, Ocelot Bio, PharmaIN, and others are developing their assets in the mid-late stage of development. With the expected approval of these therapies during the forecast period [2024–2034], the overall therapeutic market of Hepatorenal Syndrome is likely to witness a rise at a significant CAGR.
Hepatorenal Syndrome Market Insight and Trends
- The rising incidence of Hepatorenal Syndrome can be attributed to various factors, with chronic liver disease being a significant contributor.
- It was found that approximately 40% of patients with cirrhosis and ascites develop Hepatorenal Syndrome during the natural history of their disease.
- During the analysis, it was observed that Hepatorenal Syndrome acute kidney injury (AKI) accounts for approximately 11% of AKI in hospitalized cirrhotic patients with refractory ascites and is associated with high mortality.
- It was observed that the cumulative probability of developing Hepatorenal Syndrome at 1 year is approximately 18% and at 5 years is approximately 39% in patients with decompensated liver disease.
- The epidemiology of Hepatorenal Syndrome is expected to change during the forecast period (2024-2034).
- The available treatment options for Hepatorenal Syndrome, such as vasoconstrictor therapy and albumin infusion, have limitations, and not all patients respond adequately to these interventions.
Request for Sample Page @ Hepatorenal Syndrome Market Report
Factors Affecting Hepatorenal Syndrome Market Growth
Rising Prevalence of Liver Diseases
Hepatorenal syndrome primarily occurs in patients with advanced liver disease, especially cirrhosis and acute liver failure. Increasing prevalence of alcohol-related liver disease, viral hepatitis, and non-alcoholic fatty liver disease (NAFLD) contributes to a larger at-risk population, driving demand for diagnosis and therapeutic interventions.
Advancements in Diagnostic Practices
Improved diagnostics, including biomarkers and imaging techniques that enable early detection of renal dysfunction in liver disease patients, support timely intervention and better disease management. Enhanced screening in high-risk populations can boost utilization of care pathways and treatments.
Development of Novel Therapeutics
Ongoing research into targeted therapies such as vasoconstrictors (e.g., terlipressin), albumin administration strategies, and other pharmacologic agents expands treatment options. Pipeline innovation and clinical trial activity for more effective or safer agents stimulate market interest and future growth.
Healthcare Infrastructure and Access
Availability of advanced liver care, nephrology support, intensive care units, and transplantation services affects how HRS is managed. Regions with strong healthcare facilities see higher diagnosis and treatment rates, whereas limited infrastructure in developing areas restrains market penetration.
Awareness and Education
Greater awareness among clinicians and patients about hepatorenal syndrome’s high morbidity and mortality promotes early recognition and management. Educational initiatives improve adherence to diagnostic criteria and evidence-based treatment, enhancing market demand.
Reimbursement and Healthcare Funding
Coverage for expensive treatments, hospitalization, albumin therapy, and transplant options significantly influences patient access. Favorable reimbursement policies and government healthcare funding support broader adoption, while cost barriers slow uptake in some regions.
Competing Therapeutic Landscape
Competition among existing pharmacologic agents, supportive care protocols, and liver transplantation as a definitive option impacts how new treatments are adopted. Cost, efficacy, and safety profile differences influence prescribing behavior and market share allocation.
Safety and Efficacy Concerns
Treatment choices are influenced by the balance between benefits and potential adverse effects. Drugs with unfavorable safety profiles may hinder adoption, whereas therapies with strong efficacy and tolerability support growth.
DelveInsight’s comprehensive report titled “Hepatorenal Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Hepatorenal Syndrome. The report presents historical and projected epidemiological data covering Total Incident Cases of Hepatorenal Syndrome further segmented by Type and Comorbidity. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The Hepatorenal Syndrome market report analyzes the existing treatment practices and unmet medical requirements in Hepatorenal Syndrome. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Scope of the Hepatorenal Syndrome Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
Hepatorenal Syndrome Market |
|
|
Hepatorenal Syndromes Market Size | |
|
Hepatorenal Syndrome Companies |
Cumberland Pharmaceuticals, Mallinckrodt, Ferring Pharmaceuticals, Ocelot Bio Inc., Novartis, and others. |
|
Hepatorenal Syndrome Epidemiology Segmentation |
|
Hepatorenal Syndrome Disease Understanding
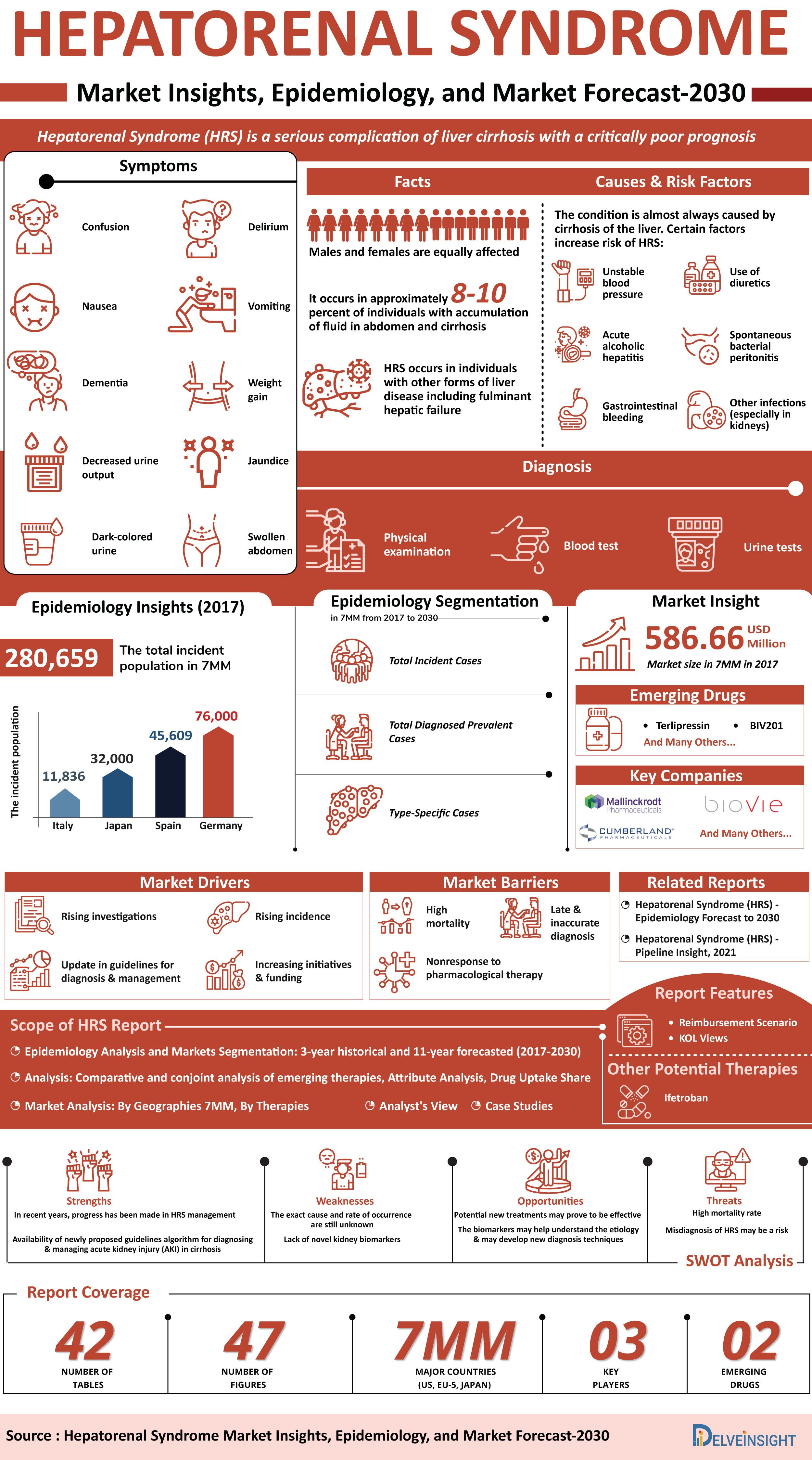
There is no specific test for Hepatorenal Syndrome. So, it is diagnosed in part by ruling out other causes of acute kidney impairment in patients with advanced liver disease. Diagnosis of Hepatorenal Syndrome involves clinical evaluation, a detailed patient history, and a variety of specialized tests.
The International Ascites Club, an organization dedicated to encouraging scientific research into advanced cirrhosis, has established criteria for a diagnosis of Hepatorenal Syndrome.
The major criteria for diagnosing Hepatorenal Syndrome include the presence of advanced liver failure with portal hypertension, high levels of creatinine (an organic acid) in the blood, absence of other causes of renal failure such as bacterial infection, shock, and the use of drugs that are toxic to the kidneys, no improvement in renal function with the withdrawal of diuretics and expansion of plasma with albumin (a protein made in the liver which is low in patients with liver disease), and low levels of protein in the urine with no evidence of urinary tract disease (uropathy) or parenchymal renal disease.
The mainstay of treatment remains vasopressor therapy with albumin to reverse splanchnic vasodilation and improve RBF. Vasoconstrictor therapy causes constriction of splanchnic vessels, resulting in increasing the effective circulating blood volume, which increases renal perfusion and glomerular filtration. Vasoconstrictors are often combined with albumin to improve their clinical benefits. Vasoconstrictors used for Hepatorenal Syndrome management are terlipressin, noradrenaline, and the combination of midodrine + octreotide.
In the US, albumin is frequently used to control Hepatorenal Syndrome symptoms. In cases where these treatments cannot be used or are ineffective, beta-blockers, rifaximin, and somatostatin are administered.
Norepinephrine, a catecholamine with predominantly midodrine, and octreotide alpha-adrenergic activity is an inexpensive alternative and widely used as an infusion for Hepatorenal Syndrome treatment. Midodrine (an α1-agonist drug) is usually administered in combination with octreotide (a somatostatin analog) and albumin, and it represents the current standard of care in the US.
Other interventions such as renal replacement therapy, transjugular intrahepatic portosystemic shunt (TIPS), and artificial liver support systems have a very limited role in improving outcomes in Hepatorenal Syndrome. Liver transplantation remains the definitive treatment for Hepatorenal Syndrome.
Hepatorenal Syndrome Overview
According to the American Liver Foundation, Hepatorenal syndrome (Hepatorenal Syndrome) is a life-threatening condition that affects kidney function in people with advanced liver disease. Hepatorenal Syndrome is most common in people with advanced cirrhosis (or scarring of the liver) and ascites, an abnormal buildup of fluid in the abdomen that is often related to liver disease. However, the syndrome can also occur in people with fulminant hepatic failure (acute liver failure) and other types of liver diseases.
Hepatorenal Syndrome involves the development of renal failure in patients with severe liver disease. Hepatorenal Syndrome is of two types: type 1 and type 2. Type 1 (Acute) involves a rapid decline in kidney function and can quickly progress to life-threatening kidney failure. Type 2 involves a more gradual decrease in kidney function. Type 2 often leads to an abnormal buildup of fluid in the abdomen (ascites) that is resistant to treatment with diuretics.
The exact cause of Hepatorenal Syndrome is unknown. The hallmark of Hepatorenal Syndrome is intense renal vasoconstriction that starts at an early time point and progresses with the worsening of the liver disease. The underlying mechanisms involved in Hepatorenal Syndrome are incompletely understood but may include both increased vasoconstrictor and decreased vasodilator factors acting on the renal circulation. Type 2 Hepatorenal Syndrome is gradually progressive and arises in association with the progression of cirrhosis, whereas type 1 is an acute deterioration in kidney function associated with severe renal vasoconstriction and failure of compensatory mechanisms responsible for the maintenance of renal perfusion.
Viral hepatitis is the most common cause of liver failure, including Hepatorenal Syndrome. Most commonly, it is caused by Hepatitis B or, less commonly, Hepatitis C. Other common causes include drugs/medications, most commonly acetaminophen, chronic alcoholism, or any drugs that induce cytochrome P450, as well as non-alcoholic steatohepatitis (NASH). Less common causes of liver failure leading to Hepatorenal Syndrome include viruses such as CMV, HHV6, and Parvovirus B19.
A variety of nonspecific symptoms are associated with Hepatorenal Syndrome which include fatigue, abdominal pain, and a general feeling of illness (or malaise) among others. Patients with Hepatorenal Syndrome may also suffer from symptoms related to advanced liver disease including the accumulation of fluid in the abdomen (ascites), yellowing of the skin and the whites of the eyes (jaundice), an enlarged spleen (splenomegaly), and an enlarged, extremely tender liver (hepatomegaly).
Signs of declining kidney function may include a significant reduction in urination, confusion, swelling caused by the buildup of fluid between tissues and organs (a condition known as edema), and abnormally high levels of nitrogen-rich, body-waste compounds in the blood (a condition known as azotemia).
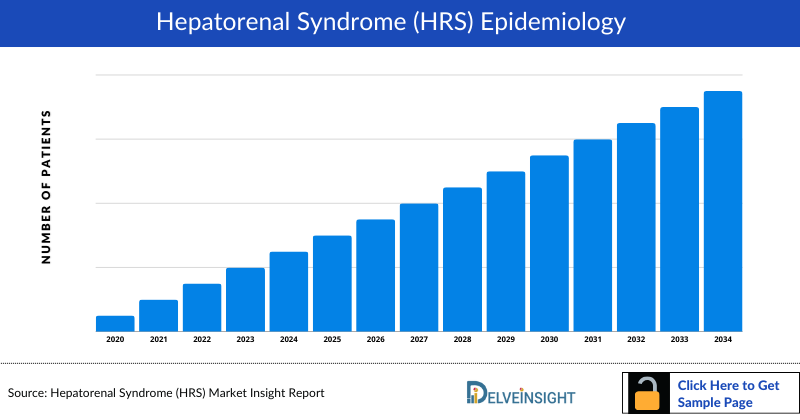
Hepatorenal Syndrome Epidemiology
The epidemiology section of the Hepatorenal Syndrome market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the incidence of Hepatorenal Syndrome. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings from Hepatorenal Syndrome Epidemiological Analysis and Forecast
- During the Hepatorenal Syndrome analysis, it was found that Hepatorenal Syndrome develops in patients with advanced cirrhosis so they will usually have jaundice and other stigmata of chronic liver disease such as finger clubbing, palmar erythema, and spider naevi.
- It was found that approximately 40% of patients with cirrhosis and ascites develop Hepatorenal Syndrome during the natural history of their disease.
- During the analysis, it was observed that Hepatorenal Syndrome acute kidney injury (AKI) accounts for approximately 11% of AKI in hospitalized cirrhotic patients with refractory ascites and is associated with high mortality.
- It was observed that the cumulative probability of developing Hepatorenal Syndrome at 1 year is approximately 18% and at 5 years is approximately 39% in patients with decompensated liver disease.
- The epidemiology of Hepatorenal Syndrome is expected to change during the forecast period (2024-2034).
Explore the latest insights and forecasts on Hepatorenal Syndrome Epidemiology for 2032. Don't miss out, delve in now!
Hepatorenal Syndrome Market Outlook
The Hepatorenal Syndrome therapeutics market is further expected to increase by the major drivers, such as the rising incident population, technological advancements, and upcoming therapies in the forecast period [2024–2034].
In September 2022, the US FDA granted approval to TERLIVAZ (terlipressin) for the treatment of adults hospitalized with Hepatorenal Syndrome with a rapid reduction in kidney function (Hepatorenal Syndrome-1). Before the approval, no approved treatment for this condition existed in the US. It is also marketed in Europe for the treatment of Hepatorenal Syndrome.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Hepatorenal Syndrome market in the 7MM is expected to change significantly during the study period 2020–2034.
Hepatorenal Syndrome Drug Analysis
Hepatorenal Syndrome Marketed Drugs
TERLIVAZ (terlipressin): Mallinckrodt Pharmaceuticals
TERLIVAZ, developed by Mallinckrodt Pharmaceuticals, is a vasopressin receptor agonist indicated to improve kidney function in adults with Hepatorenal Syndrome with a rapid reduction in kidney function. TERLIVAZ is the first and only FDA-approved product indicated for the treatment of adults with Hepatorenal Syndrome involving a rapid reduction in kidney function. Terlipressin is recommended by the American Association for the Study of Liver Diseases (AASLD) guidance and the American College of Gastroenterology (ACG) guidelines
Note: Detailed marketed therapies assessment will be provided in the final report...
Hepatorenal Syndrome Emerging Drugs
The Hepatorenal Syndrome market is expected to experience gradual changes, mainly due to the limited availability of emerging therapies in this area. Key Hepatorenal Syndrome companies, including Noorik Biopharmaceuticals, Ocelot Bio, and others have demonstrated a keen interest in this condition and are actively pursuing the development of potential treatments.
Ambrisentan: Noorik Biopharmaceuticals
Ambrisentan (N-003), an endothelin-A-receptor-antagonist is being developed by Noorik Biopharmaceuticals for the treatment of portal hypertension and associated hepatorenal syndrome with functional acute renal failure in patients with liver cirrhosis and delayed graft function (DGF). Ambrisentan can block the effects of endothelin on both the ETA and ETB receptors. Currently, the drug is in Phase II of clinical development for the treatment of Hepatorenal Syndrome.
OCE-205: Ocelot Bio
OCE-205 is a peptide therapeutic with a differentiated OCE-205 mechanism of action (MOA) designed to selectively target serious hemodynamic complications that are the result of liver fibrosis and portal hypertension in end-stage liver disease (ESLD). The first indication being explored for OCE-205 is Hepatorenal Syndrome-AKI. OCE-205’s innovation emanates from its design as a mixed agonist-antagonist peptide selective for the vasopressin 1a (V1a) receptor with no vasopressin 2 (V2) receptor activity at therapeutic concentrations. Currently, the drug is in Phase II of clinical development for the treatment of Hepatorenal Syndrome-AKI.
In August 2022, the US FDA granted Orphan Drug Designation to OCE-205 for the Treatment of Hepatorenal Syndrome.
Note: Detailed emerging therapies assessment will be provided in the final report....
Hepatorenal Syndrome Market Segmentation
DelveInsight’s ‘Hepatorenal Syndrome Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Hepatorenal Syndrome market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Hepatorenal Syndrome Market Size by Countries
The Hepatorenal Syndrome market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Hepatorenal Syndrome market, primarily attributed to the country's higher incidence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Hepatorenal Syndrome Market Size by Therapies
Hepatorenal Syndrome Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is OCE-205 under the developmental pipeline of Ocelot Bio.
Note: Detailed market segment assessment will be provided in the final report...
Hepatorenal Syndrome Drugs Uptake
This section focuses on the sales uptake of potential Hepatorenal Syndrome drugs that have recently been launched or are anticipated to be launched in the Hepatorenal Syndrome market between 2020 and 2034. It estimates the market penetration of Hepatorenal Syndrome drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Hepatorenal Syndrome market.
The emerging Hepatorenal Syndrome therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Hepatorenal Syndrome treatment market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Hepatorenal Syndrome....
Hepatorenal Syndrome Market Access and Reimbursement
DelveInsight’s ‘Hepatorenal Syndrome Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Hepatorenal Syndrome.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Latest KOL Views on Hepatorenal Syndrome
To keep up with current Hepatorenal Syndrome market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the Hepatorenal Syndrome domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Hepatorenal Syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Hepatorenal Syndrome unmet needs.
What KOLs are saying on Hepatorenal Syndrome Patient Trends?
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the American Liver Foundation, Creighton University School of Medicine, SUNY Upstate University Hospital in the US, University of Barcelona in Spain, Department of Medicine – DIMED, University of Padova in Italy, among others.
“LDLT candidates with Hepatorenal Syndrome were usually admitted or transferred to our department at least 1 month before LT, except for acute liver failure cases. During pretransplant admission, all efforts were made to recover renal function
“I am not sure about the incidence of Hepatorenal Syndrome in Japan. To the best of my knowledge, so far no Japanese nationwide study has been performed. As for my institute, the incidence was 43% out of about 500 LDLT cases.”
Note: Detailed assessment of KOL Views will be provided in the full report on Hepatorenal Syndrome.....
Hepatorenal Syndrome Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Hepatorenal Syndrome Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Hepatorenal Syndrome Clinical Trial Activities
The Hepatorenal Syndrome drugs market report offers an analysis of Hepatorenal Syndrome clinical trials within Phase II and III stages and examines companies involved in developing targeted therapeutics for Hepatorenal Syndrome. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Hepatorenal Syndrome Pipeline Development Activities
The Hepatorenal Syndrome treatment market report covers information on collaborations, acquisitions and mergers, licensing, patent details, and other information for emerging Hepatorenal Syndrome therapies.
Explore groundbreaking advancements and promising treatments in our latest Hepatorenal Syndrome Pipeline Insight for 2024. Dive in now!
Scope of the Hepatorenal Syndrome Market Report
Hepatorenal Syndrome Market Report Insights
- Hepatorenal Syndrome Patient Population
- Hepatorenal Syndrome Therapeutic Approaches
- Hepatorenal Syndrome Pipeline Analysis
- Hepatorenal Syndrome Market Size and Trends
- Hepatorenal Syndrome Market Opportunities
- Impact of Upcoming Hepatorenal Syndrome Therapies
Hepatorenal Syndrome Market Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Hepatorenal Syndrome Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Hepatorenal Syndrome Market
- Hepatorenal Syndrome Drugs Uptake
Hepatorenal Syndrome Market Report Assessment
- Hepatorenal Syndrome Current Treatment Practices
- Hepatorenal Syndrome Unmet Needs
- Hepatorenal Syndrome Pipeline Product Profiles
- Hepatorenal Syndrome Market Attractiveness
- Hepatorenal Syndrome Market Drivers
- Hepatorenal Syndrome Market Barriers
Key Questions Answered In The Hepatorenal Syndrome Market Report
- How common is Hepatorenal Syndrome?
- What are the key findings of Hepatorenal Syndrome epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Hepatorenal Syndrome?
- What are the disease risk, burden, and unmet needs of Hepatorenal Syndrome?
- At what CAGR is the Hepatorenal Syndrome market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Hepatorenal Syndrome market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Hepatorenal Syndrome in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many Hepatorenal Syndrome companies are currently developing therapies for the treatment of Hepatorenal Syndrome?
Reasons to buy Hepatorenal Syndrome Market Forecast Report
- The Hepatorenal Syndrome treatment market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Hepatorenal Syndrome Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing Hepatorenal Syndrome market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current Hepatorenal Syndrome patient share based on real-world prescription data along with reported sales of current treatment in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming Hepatorenal Syndrome companies in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Hepatorenal Syndrome companies can strengthen their development and launch strategy.
Frequently Asked Questions
What are the treatment goals for Hepatorenal Syndrome?
The primary treatment goal for Hepatorenal Syndrome is to improve renal function and prevent further deterioration, thereby increasing survival and improving the quality of life for affected individuals.
What are the challenges in managing Hepatorenal Syndrome?
Managing Hepatorenal Syndrome presents several challenges. The main key challenges include rapid progression and limited treatment options.
What are the key factors driving the growth of the Hepatorenal Syndrome market?
The Hepatorenal Syndrome market is propelled by factors like increasing incidence, medical advancements, rising awareness, and the introduction of novel therapies by key pharmaceutical players. These elements fuel demand for innovative treatments, addressing unmet medical needs and driving market expansion.
How will the Hepatorenal Syndrome Market and Epidemiology Forecast Report benefit the clients?
The report will provide comprehensive insights into the current Hepatorenal Syndrome market landscape, emerging therapies, competitive dynamics, regulatory requirements, and market access considerations, enabling informed decision-making, strategic planning, and optimization of business strategies to capitalize on market opportunities and drive growth.