Liquid Biopsy in Cancer Diagnostics Market Summary
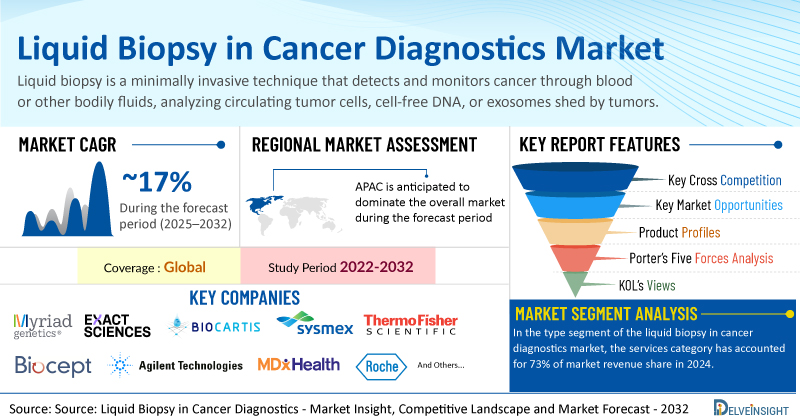
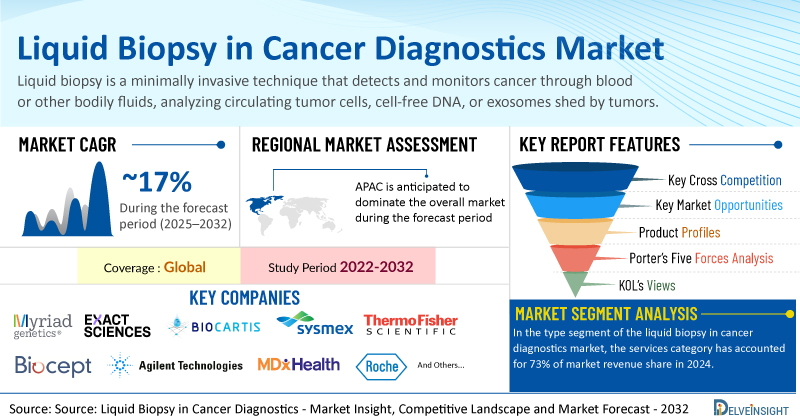
- Global liquid biopsy in cancer diagnostics market was valued at USD 7.64 billion in 2024 and expected to reach USD 19.24 billion by 2032.
- Global liquid biopsy in cancer diagnostics market is expected to grow at a CAGR of 16.64% during the forecast period from 2025 to 2032.
Global liquid biopsy in cancer diagnostics Market Trends & Insights
- The leading companies working in the Liquid Biopsy in Cancer Diagnostics Market include Myriad Genetics Inc, Exact Sciences Corporation, Biocartis, Sysmex Corporation, Thermo Fisher Scientific Inc, Biocept Inc, Agilent Technologies Inc, MDx Health, Neogenomics Laboratories, F. Hoffman La Roche Ltd, Guardant Health, Bio-Techne, Illumina Inc, QIAGEN, Lucence Health Inc, Personal Gemone Diagnostics Inc, SAGA Diagnostics, Agena Bioscience Inc, The Menarini Group, MiRXES Pte Ltd., and others.
- One of the key aspects driving the liquid biopsy in cancer diagnostics market is the growing demand for liquid biopsy in cancer diagnostics due to increase in the cancer incidence.
Global liquid biopsy in cancer diagnostics Market Size and Forecasts
- 2024 Market Size: USD 7.64 billion
- 2032 Projected Market Size: USD 19.24 billion
- Growth Rate (2025-2032): 16.64% CAGR
- Largest Market: North America
- Fastest Growing Market: Asia-Pacific
- Market Structure: Moderately Consolidated
Factors Driving the Growth of the Liquid Biopsy Market in Cancer Diagnostics
Rising Global Cancer Burden
- Increasing incidence of solid tumors such as lung, breast, colorectal, and prostate cancers is expanding the demand for non-invasive, repeatable diagnostic tools.
- Early detection initiatives and screening programs are driving adoption of liquid biopsy as a complementary diagnostic method.
Shift Toward Minimally Invasive Diagnostics
- Liquid biopsy offers a non-invasive alternative to surgical or tissue biopsy, reducing patient discomfort, complications, and cost.
- Enables frequent sampling for real-time monitoring of tumor evolution, treatment response, and resistance mutations.
Advancements in Genomic Technologies
- Rapid improvements in NGS (Next-Generation Sequencing), digital PCR, and multi-omics technologies have increased test sensitivity and specificity.
- Enhanced detection of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), exosomes, and other biomarkers supports broader applications.
Growing Use in Personalized / Precision Oncology
- Liquid biopsy enables molecular profiling, helping clinicians select targeted therapies and immunotherapies.
- Supports adaptive treatment decisions by detecting emerging mutations such as EGFR, ALK, KRAS, and BRAF.
Increasing Adoption in Treatment Monitoring
- Used increasingly for minimal residual disease (MRD) detection, relapse prediction, therapy monitoring, and identifying drug resistance.
- Offers clinicians early insights before radiological or clinical manifestations, improving patient management outcomes.
Expanding Regulatory Approvals & Reimbursement Coverage
- Multiple liquid biopsy assays have received regulatory approvals (e.g., FDA-approved cancer companion diagnostics).
- Gradual improvement in reimbursement frameworks is supporting wider clinical adoption in the US, EU, and APAC.
Integration Into Clinical Trials & Drug Development
- Pharma companies use liquid biopsies for patient selection, biomarker-based stratification, and monitoring therapeutic effectiveness.
- Increasing incorporation in oncology clinical trials is accelerating market penetration.
Rising Investments and Collaborations
- Significant funding from biotech companies, research institutions, and investors is accelerating innovation.
- Collaborations between diagnostics companies, hospitals, and pharma firms are expanding test availability and validation.
Rapid Growth of Multi-Cancer Early Detection (MCED) Tests
- MCED blood tests based on DNA methylation, cfDNA patterns, and proteomics are emerging as a major growth frontier.
- Early detection programs and pilot projects globally are adding new market opportunities.
Adoption of AI and Bioinformatics
- AI/ML algorithms improve biomarker detection, variant calling, and data interpretation.
- Advanced analytics allow rapid identification of actionable mutations and cancer-specific signatures.
Liquid Biopsy in Cancer Diagnostics Market By Type (Product Type [Instruments And Reagents & Kits] and Services), Liquid Biopsy in Cancer Diagnostics Market By Sample Type (Blood, Urine, and Saliva), Liquid Biopsy in Cancer Diagnostics Market By Biomarker Type (Circulating Tumor Cells, Circulating Tumor DNA, Circulating Free DNA, and Others), Liquid Biopsy in Cancer Diagnostics Market By Cancer Type (Lung Cancer, Breast Cancer, Colon Cancer, and Others), Liquid Biopsy in Cancer Diagnostics Market By Technique (Polymerase Chain Reaction and Next Generation Sequencing), Liquid Biopsy in Cancer Diagnostics Market By End-User (Hospitals, Diagnostic Centers, and Others), and Liquid Biopsy in Cancer Diagnostics Market By Geography is expected to grow at a steady CAGR forecast till 2030 owing to increasing Liquid Biopsy in Cancer Diagnostics prevalence of cancers and growing popularity of precision medicine.
Global liquid biopsy in cancer diagnostics market was valued at USD 7.64 billion in 2024, growing at a CAGR of 16.64% during the forecast period from 2025 to 2032 to reach USD 19.24 billion by 2034. Factors such as the rising incidence of various cancers, growing demand for minimally invasive procedures, increasing demand of precision medicine, technical innovation in product development among other factors are expected to drive the liquid biopsy in cancer diagnostics market.
Liquid Biopsy in Cancer Diagnostics Market Dynamics
For instance, as per the GLOBOCAN study conducted by the International Agency for Research on Cancer, in 2020, an estimated number of 19.3 million new cancer cases (18.1 million excluding non-melanoma skin cancer) and near about 10.0 million cancer deaths were reported globally. The above-mentioned source further stated that in 2020, lung cancer remained the leading cause of cancer death, with an estimated 1.8 million deaths (18%), followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers. Considering the increase in the number of new cancer cases all over the globe, there has been a growing emphasis on developing an alternative to conventional biopsies to facilitate the cancer diagnosis process and make it smoother for both patients and healthcare providers. Furthermore, the application of liquid biopsy in therapy management for cancers such as lung and breast is further expected to boost the product demand. Thus, the increasing patient population of cancers would, in turn, lead to an increase in demand for liquid biopsy products and services.
Furthermore, another key contributor in the growing demand for liquid biopsy in cancer diagnostics is the increasing prominence of precision medicine in cancer care. The concept of “precision medicine” or individualizing the treatment plan according to the biological behavior of the tumor is considered a new approach in cancer management. The clinical applications of precision medicine are diverse encompassing screening, diagnosis, prognosis, prediction of treatment response and resistance, early detection of recurrence/metastasis, and biologic cancer stratification. Liquid biopsies hold great promise for personalized medicine due to their ability to provide multiple non-invasive global snapshots of the primary and metastatic tumors.
The customization of treatment specific to the patient’s health condition has gained widespread acceptance and attention from physicians. Liquid biopsy plays an important role in developing customized treatments for patients. It provides vital real-time information about the tumor profile and helps decide the subsequent steps in the treatment. The potential of liquid biopsy is also being realized by market players, which is evident by their growing interest in product development in this domain. For instance, in September 2021, German firm Molecular Health GmbH collaborated with a South Korean startup EONE-Diagnomics Genome Center (EDGC) to help its healthcare provider customers identify personalized treatment options for cancer patients. Therefore, the growing prominence of precision medicine and the advantages of liquid biopsy are expected to contribute to the growth of liquid biopsy in the cancer diagnostics market during the forecast period.
Therefore, all the afore-mentioned factors will drive the market of liquid biopsy in the cancer diagnostics during the forecast period from 2025-2032.
However, risk of false results due to procedural limitations and lack of standardization of procedures may be certain challenging aspects for the liquid biopsy in cancer diagnostics market growth.
Liquid Biopsy in Cancer Diagnostics Market Segment Analysis:
Liquid Biopsy in Cancer Diagnostics Market by Type (Product Type [Instruments and Reagents & Kits] and Services), Liquid Biopsy in Cancer Diagnostics Market By Sample Type (Blood, Urine, and Saliva), Liquid Biopsy in Cancer Diagnostics Market By Biomarker Type (Circulating Tumor Cells, Circulating Tumor DNA, Circulating Free DNA, and Others), Liquid Biopsy in Cancer Diagnostics Market By Cancer Type (Lung Cancer, Breast Cancer, Colon Cancer, and Others), Liquid Biopsy in Cancer Diagnostics Market By Technique (Polymerase Chain Reaction and Next Generation Sequencing), Liquid Biopsy in Cancer Diagnostics Market By End-User (Hospitals, Diagnostic Centers, and Others), and Liquid Biopsy in Cancer Diagnostics Market By Geography (North America, Europe, Asia-Pacific, and Rest of the World)
In the type segment of the liquid biopsy in cancer diagnostics market, the services category has accounted for 73% of market revenue share in 2024. This can be ascribed to various aspects of services catered by this category. Many companies are operating in the domain where they offer services pertaining to liquid biopsy ranging from sample collection to NGS analysis. One such example is an Australia-based company, BARD1 that offers a sample preparation platform called NETs Biomarker Capture Platform. This platform is a patented technology of the company to revolutionize the performance of liquid biopsy diagnostic assays.
Another such company catering to the services aspect of the liquid biopsy domain is Biodesix. The company offers a wide range of services from clinical aspects to clients from a research background. For instance, the company employs blood-based, multiple technologies approaches for diagnostic research to uncover novel insights about the tumor biology and patient’s immune response to cancer for biopharmaceutical therapeutics in clinical development.
Therefore, considering the wide application area of services aspect of the liquid biopsy domain, the service category is expected to account for a formidable market share in the liquid biopsy in the cancer diagnostics market.
Liquid Biopsy in Cancer Diagnostics Regional Analysis
North America is expected to dominate the overall Liquid Biopsy in Cancer Diagnostics Market
Among all the regions, North America Liquid Biopsy in Cancer Diagnostics Market is expected to account for the significant Liquid Biopsy in Cancer Diagnostics market share in the liquid biopsy in cancer diagnostics market. North America is estimated to account for the dominant market share because of the increasing prevalence of cancers, increasing focus on oncology research, supportive government policies promoting liquid biopsy research and a conducive environment for product development and launches among other factors in the region.
Among all the North American countries, United States accounted for 87% of revenue share in the North America liquid biopsy in cancer diagnostics market in 2024. One of the key reasons for high uptake of liquid biopsy products and services in the country was the high prevalence of cancers.
For instance, according to the data provided by the National Cancer institute (United States), till April 2021, bladder, breast, colon and rectal, endometrial and kidney cancer were some of the common cancer types in the country. Besides, the high prevalence of Cancer, United States is considered as one of the key countries that invests heavily in healthcare services as well as medical research. The US Food and Drug Administration also provides a supportive environment for new technologies to reach the market with the provision of “breakthrough device” designation to emerging technologies and products in the medical devices domain. For instance, in April 2021, the US FDA awarded the breakthrough device designation to Inviata Limited for their liquid biopsy product for minimal residue disease (MRD) test. The regulatory body also awarded the same designation to Bluestar Genomics’ noninvasive pancreatic cancer detection test.
Additionally, cancer diagnosis and treatment can be extremely cost intensive for patients even after medical insurance. The coverage of liquid biopsy test in insurance and reimbursement programs offered by the US government further acts as a booster for their uptake by end users. For instance, InVisionFirst®, Liquid Biopsy for Patients with Lung Cancer is covered under CMS National Coverage Policy. Thus, all the above-stated factors are expected to drive the market for liquid biopsy in cancer diagnostics in the United States.
Moreover, the increasing prevalence of lifestyle disorders that present as major risk factors in cancer development. For instance, as per the data provided by the Global Obesity Observatory (2022), Mexico presents a national risk of obesity development on a scale of 10 to be 8. This represents that the majority of the population in Mexico either falls in the bracket of the obese population or is highly susceptible to being obese in the coming years. Many studies have established that excess body fat increases the risk for several cancers, including colorectal cancer, postmenopausal breast, uterine, esophageal, kidney, and pancreatic cancers. Additionally, the increase in aging population in the country is also said to contribute to cancer incidence. According to the 2021 data published by the World Bank Group, approximately 9,822,231 population in Mexico were aged 65 years and above in 2020. Therefore, the increasing prevalence of risk factors such as obesity and aging that may play a crucial role in cancer development has also resulted in the country's increasing prevalence of various cancers. This has been a major driving factor for the growing demand for liquid biopsy products and services in Mexico.
Liquid Biopsy in Cancer Diagnostics Market Key Players:
Some of the key Liquid Biopsy in Cancer Diagnostics companies operating in the liquid biopsy in cancer diagnostics market include Myriad Genetics Inc, Exact Sciences Corporation, Biocartis, Sysmex Corporation, Thermo Fisher Scientific Inc, Biocept Inc, Agilent Technologies Inc, MDx Health, Neogenomics Laboratories, F. Hoffman La Roche Ltd, Guardant Health,Bio-Techne, Illumina Inc, QIAGEN, Lucence Health Inc, Personal Gemone Diagnostics Inc, SAGA Diagnostics, Agena Bioscience Inc, The Menarini Group, MiRXES Pte Ltd., and others.
Liquid Biopsy in Cancer Diagnostics Market Recent Industry Trends and Milestones | |
|---|---|
| Category | Key Developments (2022–2025) |
| Product Launches | • Launch of next-generation liquid biopsy assays with expanded genomic panels for solid tumors. • Guardant Health released upgraded Guardant360 and GuardantINFINITY assays with enhanced tumor profiling. • Roche expanded its AVENIO blood-based NGS test kits for oncology research applications. • Emerging companies introduced early prototypes of multi-cancer early detection (MCED) liquid biopsy tests based on methylation signatures. |
| Regulatory Approvals | • FDA expanded approvals for liquid biopsy–based companion diagnostics, including new indications for Guardant360 CDx. • Natera’s Signatera MRD test received additional regulatory support for use in colorectal, breast, and other cancers. • FoundationOne Liquid CDx achieved regulatory extensions for tumor-agnostic genomic profiling. • EMA and APAC regulators increased acceptance of liquid biopsy assays in clinical oncology workflows. |
| Partnerships & Collaborations | • Diagnostics firms partnered with pharma companies to integrate liquid biopsy testing into targeted therapy trials. • AI-driven analytics companies collaborated with liquid biopsy developers to improve ctDNA interpretation. • Hospital networks and cancer centers adopted liquid biopsy MRD testing through joint programs with industry leaders. |
| Acquisitions & Investments | • Global diagnostics leaders acquired startups specializing in single-cell liquid biopsy, exosome analysis, and ultra-sensitive ctDNA platforms. • Increased venture capital investments supported multi-omics and AI-powered liquid biopsy technologies. • Companies expanded portfolios through acquisitions targeting early detection and MRD monitoring technologies. |
| Company Strategy | • Focus on expanding MRD (Minimal Residual Disease) detection across tumor types. • Strategic push toward multi-cancer early detection (MCED) test development. • Emphasis on lowering assay turnaround times, improving sensitivity, and validating tests with large-scale clinical datasets. • Efforts to increase global market penetration through payer engagement and decentralized testing models. |
| Emerging Technologies | • Advancements in methylation-based ctDNA detection and fragmentomics for early cancer detection. • Integration of multi-omics (DNA, RNA, proteins, exosomes) for comprehensive tumor profiling. • AI-enhanced bioinformatics platforms enabling high-accuracy variant calling and tissue-of-origin prediction. • Development of ultra-deep sequencing workflows and high-sensitivity MRD assays enabling earlier disease recurrence prediction. |
Recent Developmental Activities in Liquid Biopsy In Cancer Diagnostics Market:
- In October 2021, Sysmex Inostics developed a new CLIA-validated liquid biopsy test for the detection of Minimal Residual Disease (MRD) in Acute Myeloid Leukemia (AML). This new test, AML-MRD-SEQ, adds to the portfolio of ultra-sensitive Plasma-Safe-SeqS technology NGS tests available through Sysmex Inostics’ CLIA lab services in Baltimore, Maryland in the United States.
- In June 2021, NeoGenomics completed its acquisition for Inivata Ltd, a global, commercial stage liquid biopsy platform company headquartered in Cambridge, England.
- In January 2021, Exact Sciences Corporation acquired Thrive Earlier Detection Corp. (“Thrive”), a healthcare company dedicated to developing a blood-based, multi-cancer screening test.
|
Report Metrics |
Details |
|
Study Period |
2022 to 2032 |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2032 |
|
CAGR |
16.64% |
|
Liquid Biopsy in Cancer Diagnostics Market Size |
USD 19.24 billion by 2032 |
|
Liquid Biopsy in Cancer Diagnostics Companies | Myriad Genetics Inc, Exact Sciences Corporation, Biocartis, Sysmex Corporation, Thermo Fisher Scientific Inc, Biocept Inc, Agilent Technologies Inc, MDx Health, Neogenomics Laboratories, F. Hoffman La Roche Ltd, Guardant Health,Bio-Techne, Illumina Inc, QIAGEN, Lucence Health Inc, Personal Gemone Diagnostics Inc, SAGA Diagnostics, Agena Bioscience Inc, The Menarini Group, MiRXES Pte Ltd., and others |
Key Takeaways from the Liquid Biopsy in Cancer Diagnostics Market Report Study
- Liquid Biopsy in Cancer Diagnostics Market analysis for current liquid biopsy in cancer diagnostics market size (2024), and market forecast for 8 years (2025-2032)
- Top key product/services/technology developments, merger, acquisition, partnership, joint venture happened for last 3 years
- Key Liquid Biopsy in Cancer Diagnostics companies dominating the global liquid biopsy in cancer diagnostics market.
- Various opportunities available for the other competitor in the liquid biopsy in cancer diagnostics market space.
- What are the top performing segments in 2024? How these segments will perform in 2032.
- Which is the top-performing regions and countries in the current liquid biopsy in cancer diagnostics market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for liquid biopsy in cancer diagnostics market growth in the coming future?
Target Audience who can be benefited from this Liquid Biopsy in Cancer Diagnostics Market Report Study
- Liquid Biopsy in Cancer Diagnostics products providers
- Research organizations and consulting Liquid Biopsy in Cancer Diagnostics manufacturers
- Liquid Biopsy in Cancer Diagnostics-related organizations, associations, forums, and other alliances
- Government and corporate offices
- Start-up Liquid Biopsy in Cancer Diagnostics manufacturers, venture capitalists, and private equity firms
- Distributors and Traders dealing in liquid biopsy in cancer diagnostics
- Various End-users who want to know more about the liquid biopsy in cancer diagnostics market and latest technological developments in the liquid biopsy in cancer diagnostics market.
Frequently Asked Questions for Liquid Biopsy in Cancer Diagnostics Market:
1. What is Liquid Biopsy?
A liquid biopsy is a test that enables the diagnosis or analysis of tumors using only a blood or fluid sample rather than a solid tissue biopsy. It is most commonly applied to the collection of peripheral blood for analysis of cell-free circulating tumor deoxyribonucleic acids (DNA).
2. What is the market for Global Liquid Biopsy in Cancer Diagnostics?
Global liquid biopsy in cancer diagnostics market was valued at USD 7.64 billion in 2024, growing at a CAGR of 16.64% during the forecast period from 2025 to 2032 to reach USD 19.24 billion by 2032.
3. What are the drivers for Global Liquid Biopsy in Cancer Diagnostics Market?
Factors such as the rising incidence of various cancers, growing demand for minimally invasive procedures, increasing prominence of precision medicine, technical innovation in product development among other factors are expected to drive the liquid biopsy in cancer diagnostics market.
4. Who are the key players operating in Global Liquid Biopsy in Cancer Diagnostics Market?
Some of the key market players operating in the liquid biopsy in cancer diagnostics market include Myriad Genetics Inc, Exact Sciences Corporation, Biocartis, Sysmex Corporation, Thermo Fisher Scientific Inc, Biocept Inc, Agilent Technologies Inc, MDx Health, Neogenomics Laboratories, F. Hoffman La Roche Ltd, Guardant Health,Bio-Techne, Illumina Inc, QIAGEN, Lucence Health Inc, Personal Gemone Diagnostics Inc, SAGA Diagnostics, Agena Bioscience Inc, The Menarini Group, MiRXES Pte Ltd., and others.
5. Which region has the highest share in Liquid Biopsy in Cancer Diagnostics market?
North America is estimated to account for the dominant market share because of the increasing prevalence of cancers, increasing focus on oncology research, supportive government policies promoting liquid biopsy research and a conducive environment for product development and launches among other factors in the region.
Read insight blogs @ DelveInsight Blogs