Which Pharma Companies are Going to Transform the Post-Traumatic Stress Disorder Treatment Market?
Sep 25, 2025
Table of Contents
Post-Traumatic Stress Disorder (PTSD) is a complex physical, cognitive, emotional, and behavioral consequence of psychological trauma. It is characterized by intrusive thoughts, nightmares, flashbacks to past traumatic events, avoidance of traumatic reminders, hypervigilance, and sleep disturbance, all of which contribute to significant social, occupational, and interpersonal dysfunction. Post-traumatic stress Disorder causes include feelings like intense fear, helplessness, and horror.
Post-Traumatic Stress Disorder symptoms can occur as soon as a month after a stressful experience, but they can also appear years later. These Post-Traumatic Stress Disorder symptoms generate substantial issues in social and work circumstances, as well as in relationships. They can also impair one’s capacity to carry out routine everyday activities. Post-Traumatic Stress Disorder symptoms are divided into four types: intrusive recollections, avoidance, unfavorable changes in thinking and mood, and changes in physical and emotional reactions. Post-Traumatic Stress Disorder symptoms may vary from person to person and over time.
Downloads
Click Here To Get the Article in PDF
The doctor will most likely perform a physical exam to rule out any medical issues that may be causing the symptoms, a psychological evaluation that includes a discussion of the signs and symptoms, as well as the event or events that precipitated them, and will employ the criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) for the diagnosis of PTSD.
Post-Traumatic Stress Disorder Epidemiological Insights
Post-Traumatic Stress Disorder affects approximately 3.5% of adults in the US every year, and an estimated 1 in 11 people will be diagnosed with PTSD in their lifetime. Moreover, women are twice as likely as men to have Post-Traumatic Stress Disorder. According to DelveInsight’s Post-Traumatic Stress Disorder Epidemiology Forecast Report, there were approximately 5.5 million diagnosed prevalent cases of PTSD in the 7MM in 2024. This number is expected to grow by 2034, with the United States accounting for the largest share—around 5 million cases.
Current Post-Traumatic Stress Disorder Treatment Landscape
The multidimensional strategy is used in the treatment of PTSD patients. The current Post-Traumatic Stress Disorder treatment options include patient education, social support, and anxiety control through psychotherapy and psychopharmacological intervention. Patient education and social support are critical early measures for engaging the patient and mitigating the impact of the traumatic experience. Local and national support groups can assist in de-stigmatizing mental health diagnoses and emphasize that Post-Traumatic Stress Disorder symptoms are more than just a stress reaction and require Post-Traumatic Stress Disorder treatment. Family and friend support fosters understanding and acceptance, which may lessen the survivor’s guilt. Psychotherapy and counseling, therapies, or a mix of these two are commonly used in the treatment of PTSD patients.
Sertraline is also used in PTSD treatment. ZOLOFT (Sertraline) increases serotonergic activity in the central nervous system by blocking neuronal reuptake of serotonin (5-HT). It is an antidepressant that belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs). Sertraline affects brain chemicals that may be out of balance in people suffering from depression, panic attacks, anxiety, or obsessive-compulsive disorder.
Furthermore, the mechanism of action of PAXIL (Paroxetine) in PTSD treatment is unknown; however, it is thought to be related to the potentiation of serotonergic activity in the central nervous system caused by inhibition of neuronal reuptake of serotonin (5-hydroxy-tryptamine, 5-HT). Neither of these drugs is very effective in the treatment of Post-Traumatic Stress Disorder. The lack of therapies in the market enables physicians to rely on and prescribe off-label therapies with indeterminate efficacy.
Post-Traumatic Stress Disorder Market Insights
DelveInsight estimates a surge in the post-traumatic stress disorder market size owing to the rising prevalence of PTSD and growing initiatives to create public awareness and knowledge about the disease. The PTSD market in the 7MM was valued at approximately USD 1.7 billion in 2024 and is projected to increase significantly by 2034, expanding at a notable CAGR. The emergence of late-stage pipeline therapies is one of the key drivers reshaping the post-traumatic stress disorder treatment market.
One of the major growth factors is the introduction of novel drugs and therapeutic classes designed to improve the management of post-traumatic stress disorder. Innovative pipeline candidates, such as JZP150, alongside established options like SSRIs (including paroxetine [PAXIL] with its distinct MOA), are expected to broaden the spectrum of available post-traumatic stress disorder treatments. These therapies hold the potential not only to improve outcomes but also to strengthen the belief that PTSD is curable with the right interventions, offering patients new hope.

Furthermore, the relatively less competitive landscape creates an attractive opportunity for new players to enter the PTSD therapeutics market with novel and targeted mechanisms. As post-traumatic stress disorder medications continue to evolve, and as research clarifies the pathophysiology of post-traumatic stress disorder, the market outlook is expected to remain positive, with innovative therapies likely to play a central role in improving the PTSD prognosis and expanding the overall stress disorder treatment market.
Furthermore, with a few promising therapies in the Post-Traumatic Stress Disorder pipeline, the Post-Traumatic Stress Disorder treatment market size is projected to grow. Among the emerging therapies, the most promising ones include brexpiprazole + sertraline (Otsuka Pharmaceutical), BNC210 (Bionomics), Psilocybin (Compass Pathways), and others.
Post-Traumatic Stress Disorder Emerging Therapies
Several pharmaceutical Post-Traumatic Stress Disorder companies such as Tonix Pharmaceuticals, Inc., Pop Test Oncology LLC, H. Lundbeck A/S, Bionomics Limited, Alto Neuroscience, Transcend Therapeutics, Jazz Pharmaceuticals, Otsuka Pharmaceuticals, and others are among the companies active in the development of Post-Traumatic Stress Disorder therapies. As a result, the anticipated launch of Post-Traumatic Stress Disorder treatment drugs may gain a competitive advantage in the Post-Traumatic Stress Disorder treatment market. Among the promising Post-Traumatic Stress Disorder drugs in various stages of development, some are:
TNX-102 SL (Tonix Pharmaceuticals):
TNX-102 SL is a sublingual formulation of cyclobenzaprine, designed for bedtime use to improve sleep quality while avoiding the lingering effects seen in standard oral versions. It acts on multiple receptors, including serotonin2A, α1-adrenergic, histamine-H1, and muscarinic-M1, making it potentially useful for a range of neuropsychiatric conditions. Currently in Phase III trials for PTSD, it also has an FDA Fast Track designation for use in Alzheimer’s-related agitation, and is being explored for fibromyalgia and alcohol use disorder.
Methylone / TSND-201 (Transcend Therapeutics):
Methylone is an experimental neuroplastogen that quickly activates genes related to brain plasticity, including BDNF, in brain regions involved in emotion and memory. This action may help correct circuitry deficits seen in PTSD, depression, and anxiety. It is currently being evaluated in Phase II trials as a novel therapeutic for PTSD, with the goal of providing faster and more durable symptom relief.
BXCL501 (BioXcel Therapeutics):
BXCL501 is a dissolvable film containing dexmedetomidine, a selective alpha-2 receptor agonist, developed to reduce agitation and stress-related behaviors. With its novel delivery method and mechanism, it targets symptoms common in PTSD and other neuropsychiatric conditions. BXCL501 is currently in early (Phase I) clinical development for PTSD treatment.
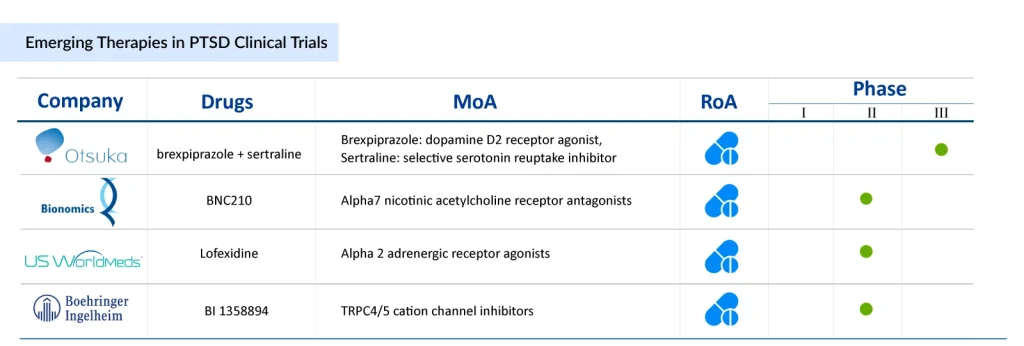
Brexpiprazole + Sertraline (Otsuka Pharmaceuticals):
This investigational combination therapy includes brexpiprazole, a serotonin-dopamine activity modulator, and sertraline (ZOLOFT), a widely used SSRI. The combo is being tested for enhanced effectiveness in managing PTSD symptoms. In early 2025, the FDA is expected to hold an advisory meeting on the drug’s approval, delaying the original decision timeline. This combination may offer a more comprehensive approach by targeting multiple neurotransmitter systems involved in PTSD.
BNC210 (Bionomics Limited):
BNC210 is a novel, non-sedating drug that modulates the α7 nicotinic acetylcholine receptor, potentially reducing anxiety without the typical side effects of benzodiazepines. It’s being developed for PTSD, social anxiety disorder, and general anxiety. In late 2024, Bionomics released positive Phase 2 results from its ATTUNE study, showing strong clinical potential. These findings were recognized in NEJM Evidence and presented at a major neuropsychopharmacology conference.
Expected Roadblocks
The post-traumatic stress disorder therapeutics market is expanding; however, several challenges continue to limit its full growth potential. One of the most critical barriers is the high prevalence of comorbidities, psychiatric, medical, and substance-related, which complicate care pathways and reduce treatment effectiveness. These overlapping conditions often worsen patient outcomes, leading to poor quality of life and increased healthcare burden, thereby restricting the overall PTSD treatment market.
The pathophysiology of post-traumatic stress disorder further complicates progress, as the disorder presents with heterogeneous symptoms and, in many cases, a delayed onset. This variability makes it difficult to standardize care and delays diagnosis, impeding timely interventions and effective management of post-traumatic stress disorder.
Additionally, the expiration of patents for several leading post-traumatic stress disorder medications, alongside the rising presence of generics, is reshaping the competitive landscape. While generics improve accessibility, they also exert downward pricing pressures on branded drugs, creating challenges for market sustainability.
Despite these hurdles, the market continues to evolve, driven by pipeline candidates such as JZP150 and other novel mechanisms targeting hyperarousal and fear extinction pathways. At the same time, established treatments like SSRIs (e.g., paroxetine, known as Paxil, with its unique MOA targeting serotonin reuptake) remain the foundation of care. However, growing awareness that PTSD is curable in some patients with early intervention and comprehensive PTSD management strategies underscores the need for continued therapeutic innovation.
Ultimately, while the post-traumatic stress disorder market faces significant barriers—from comorbidities to pricing challenges, the pursuit of advanced therapeutics offers hope for more effective stress disorder treatment and improved long-term PTSD prognosis.

FAQs
Pharma companies are focusing more on PTSD because awareness has grown around its high prevalence, its economic and social burden, and the unmet medical need left by limited first-line therapies like SSRIs. In addition, regulatory incentives such as FDA Fast Track and Breakthrough Therapy Designation are encouraging investment, while advances in neuroscience and drug delivery have opened new therapeutic possibilities.
Traditional SSRIs target serotonin pathways and often take weeks to show effect, with mixed efficacy in PTSD patients. Emerging drugs like BNC210 (a nicotinic acetylcholine receptor modulator) and JZP150 (an FAAH inhibitor that enhances endocannabinoid signaling) work through novel mechanisms aimed at faster, more durable symptom relief and fewer side effects.
Yes, psychedelic-assisted therapies are gaining traction because they target trauma memory processing and emotional rewiring in ways traditional drugs cannot. Clinical trials suggest psilocybin may deliver rapid, sustained improvements after only a few guided sessions, which could redefine treatment models if approved. Several companies are exploring this space, making it a disruptive frontier in PTSD care.
Unlike depression and anxiety, PTSD has historically lacked a wide range of approved therapies, with only a couple of SSRIs officially indicated. This has left significant gaps in the treatment landscape. The lower competition makes PTSD an attractive therapeutic area for pharma companies developing novel drugs, since even one successful launch can quickly capture strong market share.
Yes, many experimental drugs for PTSD target pathways linked to stress regulation, fear extinction, and neuroplasticity, which also play roles in traumatic brain injury (TBI), depression, and substance abuse. This overlap raises the possibility that a single therapy could be effective across multiple comorbid conditions, making these drugs more versatile and commercially viable.
Beyond symptom scores, trials now track sleep quality, functional recovery, emotional regulation, and quality-of-life outcomes reported directly by patients. These measures better capture the holistic impact of treatment, reflecting whether patients can return to work, rebuild relationships, and manage daily life — outcomes that regulators and payers increasingly value.
Downloads
Article in PDF