Post-traumatic Stress Disorder (PTSD) Treatment: Will the Emerging Therapies Succeed in Curing this Invisible Wound?
Jan 20, 2023
Table of Contents
Post-traumatic stress disorder (PTSD) is a disorder that affects a wide range of people and can be triggered by various traumatic events, despite its association with military veterans or active-duty service members. According to the Department of Veterans Affairs, approximately 12 million adults in the United States suffer from PTSD each year. The severity of the condition appears to be evenly distributed, with 36.6% of people suffering from severe symptoms, 33.1% suffering from moderate symptoms, and 30.2% suffering from mild PTSD symptoms. It primarily affects women, around 10% of women have PTSD sometime in their lives compared to 4% of men. The lifetime worldwide prevalence of PTSD in the general population is around 3.9%. In people known to have been exposed to trauma, the rate is 5.6%.
PTSD symptoms can vary in intensity; the patients might present with PTSD symptoms when they are generally stressed or reminded of their past sufferings. PTSD symptoms include intrusion, avoidance, alterations in cognition and mood, and alterations in arousal and reactivity.
Downloads
Click Here To Get the Article in PDF
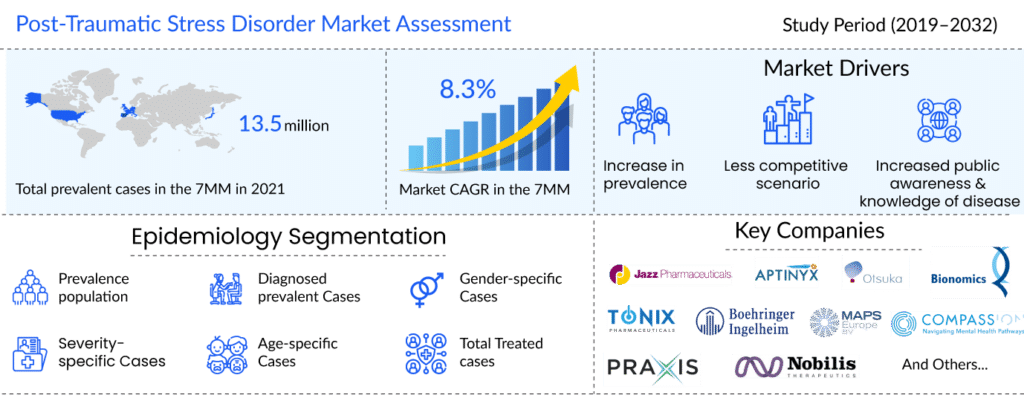
As per a recent analysis in the “PTSD Epidemiology” report by DelveInsight, in the 7MM, the total prevalent and diagnosed 12 months prevalent cases of PTSD in 2021 was approximately 13.5 million and 5.2 million cases, respectively, and are expected to grow at a significant CAGR by 2032.
The growth in the PTSD treatment market can be attributed to an increase in life stressors, traumatic events, lack of support, and increased initiatives to create public awareness and knowledge of the disease.
What are the currently available PTSD treatment options for patients?
The PTSD treatment of patients relies on a multi-dimensional approach, including patient education, social support, and anxiety management through psychotherapy and psychopharmacologic intervention. Patient education and social support are critical initial interventions to engage the patient and mitigate the impact of the traumatic event.
Across the globe, all guidelines recommend that Psychotherapy (Cognitive Processing Therapy/Prolonged Exposure Therapy/Eye Movement Desensitization, and Reprocessing/Stress Inoculation Training) should be the first line of PTSD treatment as it can help patients to resolve their disease. However, PTSD medications will only provide symptomatic management of the disease. Hence the use of pharmacotherapy is recommended only in patients with severe disease, and as per our analysis, around 35% of cases are of severe PTSD.
The most commonly used pharmacotherapies include antidepressants (SSRI, SSNRI, TCA, Tetracyclic antidepressant, SARIs) and antipsychotic drugs (risperidone, quetiapine, etc.). Currently, only paroxetine and sertraline are FDA-approved drugs for post-traumatic stress disorder treatment, which belong to the class of selective serotonin reuptake inhibitors (SSRI).
What is the unmet need for the current PTSD treatment market?
PTSD is a psychiatric disorder with significant unmet medical needs despite extensive clinical and translational research. These include the need for effective, approved PTSD medications and novel diagnostic techniques for diagnosing PTSD.
Scope of upcoming therapies in the PTSD treatment space
There is a requirement for new options in the severe patient pool (~in 30-35% of PTSD cases) where we have minimal PTSD treatment options. Therefore, the new therapies will have more opportunities and less PTSD treatment market competition.Here is a list of major key players and competitors working in the PTSD pipeline:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Apart from the above other key players like Jazz Pharmaceuticals (Nabiximols in PII/II), Tonix Pharmaceuticals (TNX-102 SL [cyclobenzaprine HCl] in PII), Boehringer Ingelheim (BI 1358894 in PII), Praxis Precision Medicines (PRAX-114 in PII), Alto Neuroscience (ALTO-100 in PII), COMPASS Pathways (COMP-360 in PII), MAPS Europe B.V. (MDMA [Methylenedioxymetamphetamine] in PII) and Nobilis Therapeutics (NBTX-001 [Xenon Inhaler] in PII), are also working in the PTSD treatment market.
What’s cooking in the PTSD treatment domain?
- In January 2023, MAPS Public Benefit Corporation, a private biopharmaceutical company dedicated to the development and commercialization of psychedelic medicines, has announced positive results from MAPP2, a multi-site Phase III study of MDMA-assisted therapy for PTSD.
- In October 2022, Acer Therapeutics Inc. announced the expansion of ACER-801 (osanetant) into a new indication for the reduction of the frequency and severity of acute stress disorder and post-traumatic stress disorder (PTSD).
- In September 2022, atai Life Sciences N.V., which is developing EMP-01, a 3,4-methylenedioxy-methamphetamine (MDMA) derivative for the treatment of post-traumatic stress disorder (PTSD) and other indications, announced it’s Phase I study has received regulatory and ethics approvals required from Medsafe and HDEC, respectively, to initiate participant enrollment.
- In May 2022, data from preclinical studies of Aptinyx’s NYX-783 in models of post-traumatic stress disorder (PTSD) were presented in a poster at the American Psychiatric Association Annual Meeting which was held from May 21 – 25, 2022, in New Orleans, LA.
- In May 2022, Beckley Psytech Limited and Lophora ApS announced the companies had entered into a research and development collaboration. Under the terms of the agreement, Beckley Psytech will jointly fund continuing development of the Lophora pipeline and collaborate broadly on R&D.
- In May 2022, The University of Nebraska Medical Center (UNMC) and ANANDA Scientific Inc announced a collaboration in a new clinical trial investigating treatment for adults with Post-Traumatic Stress Disorder (PTSD). The study will evaluate the effectiveness of Nantheia™ ATL5, an investigational drug using cannabidiol in ANANDA’s proprietary delivery technology. An investigational new drug (IND) application for the trial has been approved by the U.S. Food and Drug Administration (FDA).
- In February 2022, Novamind Inc. announced that it is enrolling participants in phase II clinical trial sponsored by Alto Neuroscience. The eight-week clinical trial currently underway at Novamind’s Draper, Utah research site is investigating an antidepressant medication for adults with major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) (the “Alto Clinical Trial”).
The optimistic future of the PTSD treatment market
The post-traumatic stress disorder market size is expected to grow at a CAGR of 8.3% from 2021 and generate approx USD 2 billion in the seven major markets (the United States, Germany, France, Italy, Spain, UK, and Japan) by 2032 due to expected approvals of the emerging therapies.
Less competitive scenarios, increasing government initiatives to create public awareness, and increasing the prevalence of PTSD patients will also positively impact the PTSD treatment market’s growth rate. Moreover, the introduction of novel drugs and classes of new therapeutic drugs for post-traumatic stress disorder treatment into the market will provide hope to PTSD patients and may have a significant positive impact on the PTSD treatment market. Furthermore, new diagnostic categories based on PTSD have been proposed, including Prolonged Duress Stress Disorder, Post-Traumatic Mourning Disorder, Post-Traumatic Relationship Syndrome, Post-Traumatic Dental Care Anxiety, and Post-Traumatic Abortion Syndrome, which will all help to boost the PTSD treatment market growth in the coming years.

FAQs
Post-traumatic stress disorder (PTSD) has been defined as “the complex physical, cognitive, emotional, and behavioral consequences of psychological trauma.” It is characterized by intrusive thoughts, nightmares, flashbacks to past traumatic events, avoidance of traumatic reminders, hypervigilance, and sleep disturbance, all of which contribute to significant social, occupational, and interpersonal dysfunction.
PTSD symptoms can occur as soon as a month after a stressful experience, but they can also appear years later. These PTSD symptoms generate substantial issues in social and work circumstances, as well as in relationships. They can also impair one’s capacity to carry out routine everyday activities.
As per a recent analysis by DelveInsight, in the 7MM, the total prevalent and diagnosed 12 months prevalent cases of PTSD in 2021 was approximately 13.5 million and 5.2 million cases, respectively, and are expected to grow at a significant CAGR by 2032.
PTSD causes include feelings like intense fear, helplessness, and horror.
Several pharmaceutical post-traumatic stress disorder companies, such as Jazz Pharmaceuticals, Aptinyx, Otsuka Pharmaceutical Development & Commercialization, Inc., Bionomics Limited, Tonix Pharmaceuticals, Boehringer Ingelheim, Praxis Precision Medicines, Alto Neuroscience COMPASS Pathways, MAPS Europe B.V., Nobilis Therapeutics, and others, are among the companies active in developing post-traumatic stress disorder therapies.
The emerging therapies in the post-traumatic stress disorder pipeline include JZP150, NYX-783, Rexulti (brexpiprazole) and Zoloft (sertraline) combination, BNC210, and others.
The doctor will most likely perform a physical exam to rule out any medical issues that may be causing the symptoms – a psychological evaluation that includes a discussion of the signs and symptoms, as well as the event or events that precipitated them, and will employ the criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) for the PTSD diagnosis.
The multidimensional strategy is used in PTSD management. The current PTSD treatment options include patient education, social support, and anxiety control through psychotherapy and psychopharmacological intervention.
There are two PTSD medicine used for PTSD treatment. These PTSD medicines include Sertraline (Zoloft) and Paxil (Paroxetine).
Downloads
Article in PDF