New Frontiers in Uveitis Treatment: Updates and Innovations
Aug 13, 2025
Table of Contents
Uveitis remains one of the leading causes of preventable vision loss worldwide, accounting for nearly 10–15% of blindness cases in developed countries. Its complex and multifactorial nature poses significant diagnostic and therapeutic challenges, demanding prompt detection and targeted intervention to preserve sight. In recent years, advances in immunomodulatory therapies, biologics, and precision diagnostics have opened new possibilities for controlling inflammation and preventing irreversible ocular damage. As research accelerates, clinicians and researchers are moving closer to more effective, personalized strategies that can address both the underlying causes and the devastating consequences of this sight-threatening condition.
What is Uveitis?
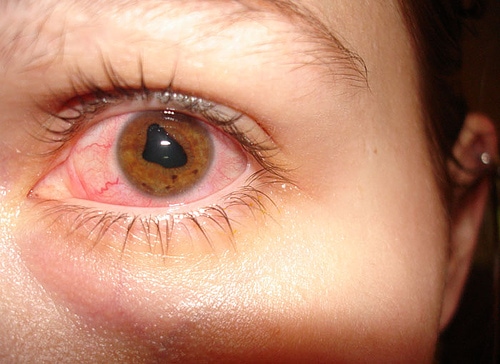
Uveitis is a serious intraocular inflammatory condition that primarily affects the uvea, the vascular middle layer of the eye, but can also involve the retina and optic nerve. It may be triggered by autoimmune eye diseases, systemic inflammatory disorders, or infections, and presents with characteristic uveitis symptoms such as eye redness, pain, photophobia, and blurred vision. Broadly, it is classified as infectious uveitis, caused by bacteria, viruses, or parasites, and noninfectious uveitis (NIU), which is often linked to systemic autoimmune conditions or confined ocular autoimmunity. NIU accounts for the majority of cases.
Downloads
Click Here To Get the Article in PDF
Clinically, uveitis is categorized based on the primary site of inflammation: anterior uveitis (most common, involving the iris, often associated with HLA-B27–related disorders), intermediate uveitis (affecting the vitreous and peripheral retina, sometimes linked to sarcoidosis or multiple sclerosis), posterior uveitis (targeting the retina and choroid, often linked to infections such as toxoplasmosis or systemic inflammatory conditions), and panuveitis (the most severe form, involving all segments of the uvea and often reflecting idiopathic or complex systemic disease).
Although the exact causes of uveitis are often unknown, it can be associated with conditions such as juvenile idiopathic arthritis (JIA), Behçet’s disease, and Vogt–Koyanagi–Harada (VKH) syndrome. If not managed promptly with appropriate uveitis treatment options, ranging from eye drops for uveitis to immunosuppressive drugs, it may lead to complications such as macular edema, cataract, or glaucoma, resulting in irreversible vision loss. Understanding uveitis prevalence, accurate classification, and timely intervention are key to preventing blindness and improving patient outcomes.
Uveitis on the Rise: Contributing Factors for a Rise in Diagnosed Prevalence
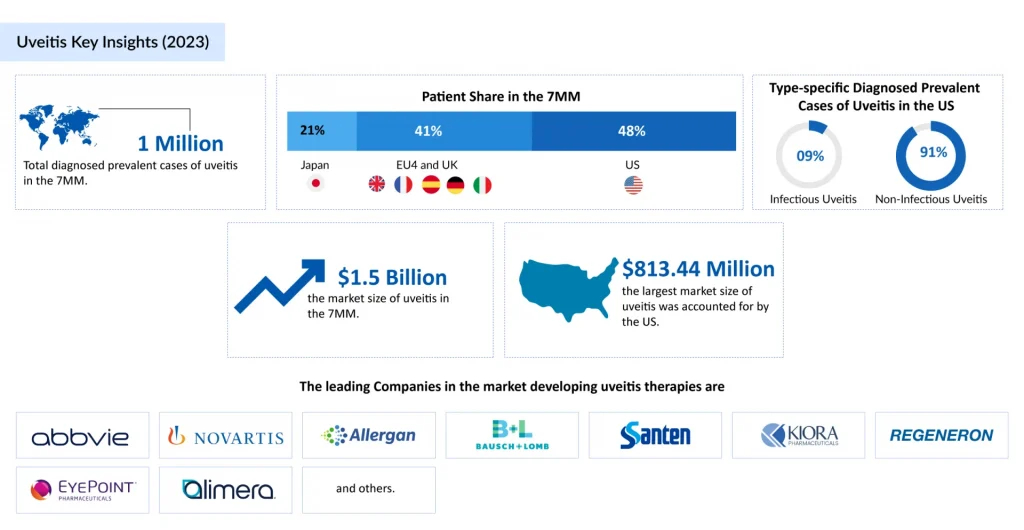
Uveitis is a rare eye condition. As greater awareness has been raised about the disease in recent years and more advanced diagnostic techniques are being employed, prevalence has expanded dramatically. DelveInsight estimates that the diagnosed prevalent cases of uveitis in the US rose to almost 400,000 in 2024. About 90% of these cases were non-infectious, and among anatomical location types, anterior NIU made up almost 70% of all uveitis cases. Idiopathic uveitis accounted for ~40% of all uveitis cases in the US.
Healthcare providers are now better equipped to detect and diagnose uveitis, allowing for earlier intervention and better outcomes. Optical coherence tomography (OCT) has played a critical role as a non-invasive imaging technique used to diagnose and monitor uveitis. Fluorescein angiography helps identify areas of inflammation and damage in the eye. Multiplex polymerase chain reaction (PCR) testing detects the presence of multiple pathogens in a single test and diagnosis of infectious causes of uveitis. Next-generation sequencing (NGS) is a high-throughput technique that rapidly sequences large amounts of genetic material. It can be used to identify genetic mutations that may contribute to the development of uveitis. Overall, these advancements in imaging and laboratory techniques have greatly improved the accuracy and efficiency of uveitis diagnosis, allowing for earlier detection.
The increasing prevalence of autoimmune diseases such as rheumatoid arthritis, lupus, multiple sclerosis, and others has also greatly contributed to the cases of uveitis as a secondary condition. Certain infections, such as herpes simplex, tuberculosis, and toxoplasmosis, may also trigger uveitis. Susceptibility to the disease is greater in the elderly as immune functions decline.
Need for Improving Disease Reporting and Surveillance Systems
Uveitis, a leading cause of visual morbidity, is estimated to account for 10–15% of blindness in the United States, underscoring its substantial clinical and socioeconomic impact. Yet, accurate epidemiological assessment remains challenging due to the disease’s heterogeneity—spanning multiple anatomical subtypes, etiology, and patient demographics. Reported incidence and prevalence figures fluctuate widely, influenced by differences in study design, diagnostic criteria, clinical settings, and population characteristics, as well as shifts in infectious disease patterns, systemic associations, and the adoption of advanced imaging or molecular diagnostics.
Addressing these limitations requires the establishment of standardized, real-time surveillance systems and contemporary, multicenter, population-based studies to capture accurate and comparable data. International uveitis registries, integrated electronic health records, and cross-border data harmonization initiatives could significantly enhance monitoring. Emerging tools such as AI-driven analytics, big-data epidemiology, and automated image recognition for anterior and posterior segment inflammation hold promise for earlier detection, more precise classification, and trend forecasting. Such advancements would not only refine burden estimates but also inform healthcare policy, optimize resource allocation, and accelerate the development of targeted prevention and therapeutic strategies.
Current Uveitis Treatment Approaches
The primary goal in uveitis management is to suppress intraocular inflammation, preserve vision, and minimize treatment-related toxicity, with strategies tailored to etiology, anatomical classification, and disease severity. Infectious causes require targeted antimicrobial or antiviral therapy before anti-inflammatory intervention, while idiopathic or autoimmune-associated cases, particularly in the treatment of posterior uveitis, follow a stepwise approach starting with corticosteroids, administered as topical drops, periocular/intravitreal injections, or oral agents, and escalating to immunosuppressants or biologics when necessary. Nonsteroidal anti-inflammatory drugs may help in milder forms, whereas immunosuppressants such as methotrexate, mycophenolate, or cyclosporine serve as steroid-sparing agents in chronic disease.
Biologics, particularly anti-TNF agents such as HUMIRA (adalimumab) and REMICADE (infliximab), have markedly advanced the management of non-infectious uveitis, offering targeted, steroid-sparing disease control. The patent expiry of these agents has opened the market to cost-effective biosimilars, such as AMJEVITA, enabling broader patient access and delivering measurable savings to healthcare systems. This shift has been supported by robust evidence: multiple pivotal and real-world studies confirm that adalimumab biosimilars achieve comparable efficacy to the originator in preventing NIU relapse, with preserved safety and tolerability profiles.
Advances in ocular drug delivery have been equally transformative, enabling targeted, sustained intraocular therapy while minimizing systemic side effects. Key innovations include OZURDEX (dexamethasone intravitreal implant), offering effective short-to-intermediate control for 3–4 months and often used in episodic or acute flares; YUTIQ (fluocinolone acetonide), providing ultra-low-dose corticosteroid release for up to 36 months, which is particularly valuable in chronic, smoldering NIU; and RETISERT (fluocinolone acetonide), a surgically implanted high-dose option with a similar 36-month duration, typically reserved for severe, vision-threatening disease requiring aggressive local steroid delivery. These implants have significantly reduced uveitis treatment burden and improved adherence but remain unsuitable for patients with glaucoma, active infection, or corticosteroid-induced ocular hypertension, underscoring the importance of patient selection.
Macular edema, seen in approximately 20–30% of uveitis patients and a leading driver of irreversible vision loss, has spurred a new wave of posterior segment–targeted therapy. XIPERE (triamcinolone acetonide injectable suspension) utilizes a suprachoroidal delivery route to achieve high retinal drug concentrations with minimal anterior segment exposure, potentially lowering the risk of steroid-induced cataract and intraocular pressure elevation. The uveitis latest treatment includes ILUVIEN (fluocinolone acetonide intravitreal implant), FDA-approved in April 2025 for chronic NIU of the posterior segment, which delivers continuous micro-dose steroid therapy for up to 36 months, offering a long-term, low-maintenance option for patients prone to relapse.
Despite therapeutic advancements, there is still no guaranteed way to cure uveitis, and many patients require a trial-and-error approach to identify the most effective uveitis treatment. Uveitis continues to have significant unmet treatment needs, as currently available therapies, though effective in many cases, may not work for every patient. All treatment options for uveitis carry potential risks: biologics increase susceptibility to infections, corticosteroids can lead to elevated eye pressure, cataracts, or glaucoma, and implant-based therapies require surgical or procedural intervention with ongoing monitoring. High drug costs, limited insurance coverage, and access barriers remain major challenges in the uveitis drug market. Injectable and implant-based eye inflammation treatments may necessitate additional clinic visits or surgery, adding inconvenience and discomfort for patients. Furthermore, those on long-term therapy require close monitoring through specialized testing to detect side effects or complications, which can be both time-consuming and costly.
“There is a requirement for enhanced initial treatment of inflammation, improved maintenance of persistent chronic inflammation, and better handling of disease complications like macular edema. Although there are some encouraging advancements like small molecules and gene therapy that are expected to tackle some unmet market demands, there is still a need for additional molecule options.” – Key Opinion Leader
From Broad Immunosuppression to Precision Therapy
The treatment of uveitis is evolving with newer drugs and innovative advances in ocular drug delivery, driven by a deeper understanding of disease pathophysiology and the challenges of effective drug penetration. The uveitis treatment market is witnessing a shift toward targeted, mechanism-based therapies that address unmet needs in patient subgroups poorly served by current options, raising hopes for more effective interventions and potentially a permanent cure for uveitis in the future. Several promising agents in uveitis clinical trials are set to reshape the landscape by improving efficacy, reducing side effects, and enhancing patient convenience.
Brepocitinib, an oral, once-daily, first-in-class dual selective TYK2/JAK1 inhibitor from Priovant Therapeutics, is in a registrational Phase III trial for NIU, and in September 2024, Priovant announced that brepocitinib has been granted Fast Track Designation from the FDA for NIU, underscoring its potential to expedite access for a condition with limited uveitis treatment diversity. The anticipated Phase III results, expected in the first half of 2027, build on encouraging NEPTUNE trial findings, which reinforce the therapeutic relevance of TYK2/JAK1 blockade in severe, immune-mediated ocular inflammation.
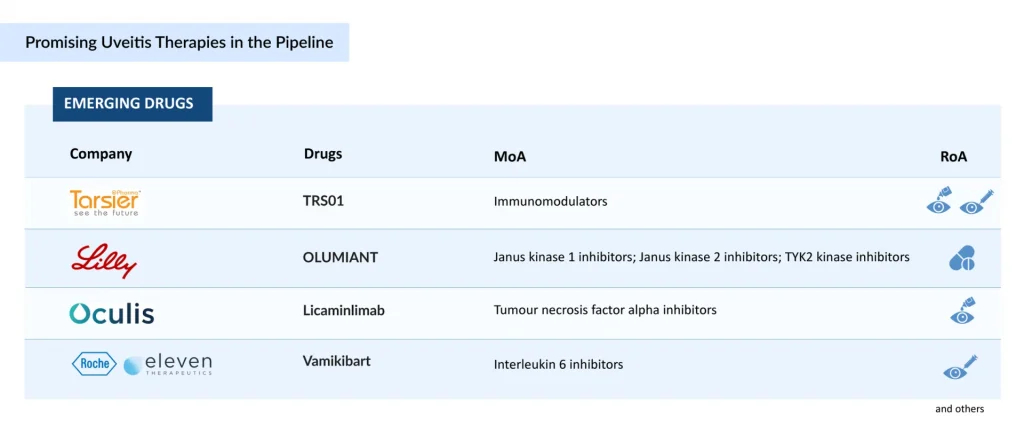
Dazdotuftide (TRS01) from Tarsier Pharma offers a differentiated, corticosteroid-sparing approach, formulated as eye drops for uveitis in patients with uveitic glaucoma—a particularly challenging cohort due to steroid-induced intraocular pressure risks. Following positive outcomes in its first Phase III study and a favorable FDA Type C meeting, TRS01 is advancing into Tarsier-04, a second Phase III trial conducted under a Special Protocol Assessment (SPA) to support regulatory submission. The therapy also holds EMA orphan drug designation and recognition for its clinically meaningful advantage in NIU patients with uveitic glaucoma who are not candidates for corticosteroid treatment.
On the biologic front, Licaminlimab (OCS-02), a topical anti–TNF-α fragment, has shown positive Phase II efficacy in acute anterior uveitis but lacks current active development for uveitis, with company’s focus now shifted to dry eye disease.
Together, these agents exemplify a pipeline that is evolving beyond broad immunosuppression toward mechanism-driven, targeted, and patient-tailored interventions, with the potential to improve long-term vision preservation while reducing treatment-related risks.
FAQs
Uveitis is a rare condition in which all or part of the uvea becomes inflamed. The uvea includes the choroid, the ciliary body, and the iris. The disease is commonly associated with visual impairment, blindness, and decreased quality of life.
Early uveitis symptoms may be mild or severe, depending on which part of the uveal tract is affected and the amount of inflammation. Anterior uveitis symptoms are usually the most bothersome. They may include severe eye pain, redness of the conjunctiva, sensitivity to light, decreased vision, the presence of white blood cells floating in the aqueous humor, the accumulation of white blood cells on the inner surface of the cornea, and other symptoms.
A comprehensive eye exam is required for uveitis diagnosis. The clinical diagnosis is frequently predicated on spillover inflammation (i.e., cell and protein flare) visible with a slit lamp in the aqueous or vitreous humor.
Uveitis treatment seeks to bring the disease to a halt. Corticosteroids are frequently used as the first-line therapy to decrease inflammation. In addition to corticosteroids, immunosuppressant therapy, antibiotics or antivirals, biologics, and other medicines are employed.
The observed increase in uveitis cases reflects both a true rise in prevalence and advancements in detection. Improved diagnostics, enhanced interdisciplinary collaboration, and heightened clinical awareness have reduced the proportion of idiopathic cases, uncovering more instances linked to autoimmune disorders, infectious etiologies such as ocular tuberculosis and viral retinitis, and age-associated conditions including herpetic iridocyclitis.
Growth in the uveitis treatment market is driven by rising disease prevalence, particularly linked to autoimmune disorders, infections, and aging populations, alongside increased recognition through improved diagnostics. Advances in biologics, biosimilars, and sustained-release delivery systems are reshaping treatment paradigms, supported by robust R&D pipelines and expanding clinical trial activity.
The optimal treatment for uveitis depends on the underlying cause, severity, and location of inflammation. First-line management typically involves corticosteroids, administered topically, orally, via periocular injection, or as intraocular implants, to rapidly control inflammation. In recurrent, severe, or steroid-refractory cases, immunomodulatory therapies such as methotrexate, mycophenolate mofetil, or biologics targeting TNF-α or IL-6 are employed to achieve long-term control while minimizing steroid dependence. Prompt diagnosis, targeted therapy, and close multidisciplinary monitoring are essential to preserve vision and prevent complications.
Currently, there is no guaranteed way to cure uveitis, as treatment primarily focuses on controlling inflammation, preserving vision, and preventing recurrences. While various treatment options for uveitis, such as corticosteroids, immunosuppressants, and biologic agents, can effectively manage eye inflammation, their efficacy varies among patients, often requiring a trial-and-error approach.

Downloads
Article in PDF