How the Amniotic Membrane Market is Transforming Regenerative Healthcare Worldwide
Jul 02, 2025
Table of Contents
The amniotic membrane market is revolutionizing healthcare by harnessing the natural healing power of the amniotic membrane, a thin placental layer renowned for its regenerative properties. This remarkable biomaterial is transforming treatments across specialties, particularly in amniotic membrane eye treatment, where it serves as a game-changer for conditions like dry eye and corneal damage. With over 80,000 amniotic membrane grafts used annually in the U.S. alone, these membranes are celebrated for their anti-inflammatory and antimicrobial qualities, promoting faster tissue repair with minimal amniotic membrane eye side effects.
From amniotic membrane eye procedures to wound care and orthopedics, this market is driving innovation, offering hope for patients seeking effective, non-invasive solutions like amniotic membrane for dry eye. This blog explores the dynamic applications and advancements propelling the amniotic membrane market into the future of regenerative medicine.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Neuromodulation Devices Observed to Reshape the Migraine Treatment Dynamics
- FDA Approves Sanofi/Regeneron’s DUPIXENT as First New CSU Therapy in Over a Decade; Gilead’s TROD...
- FDA Clearance to iRhythm’s ZEUS System; Respiri Introduces the Wheezo Device & App; CONMED to...
- Relievant Medsystems Launched Ablation System; Thermo Fisher’s Reproductive Health Assays; EarliT...
- Upcoming Contenders in Dry Eye Disease Drug Space: 5 Therapies to Watch Post-TRYPTYR Approval
What is the Amniotic Membrane?
The amniotic membrane is the innermost layer of the placenta, a thin, transparent tissue that protects the fetus during pregnancy. Rich in growth factors, anti-inflammatory agents, and antimicrobial properties, it promotes healing and tissue regeneration, driving its prominence in the amniotic membrane market. Processed into cryopreserved or lyophilized forms, it serves as a biological graft or bandage for applications like amniotic membrane eye treatment and wound care. Its ability to reduce scarring and support recovery with minimal amniotic membrane eye side effects makes it ideal for amniotic membrane eye procedures, such as treating dry eye or corneal damage, cementing its role in regenerative medicine.
Key Applications Driving the Amniotic Membrane Market
The amniotic membrane market thrives on its versatile applications across medical fields:
Ophthalmology: Amniotic membrane for eye treatments excels in repairing corneal ulcers, treating amniotic membrane for dry eye, and supporting pterygium surgery, with amniotic membrane eye procedures reducing inflammation and promoting healing.
Wound Care: Amniotic membranes treat chronic wounds, such as diabetic foot ulcers and burns, by reducing inflammation and preventing infections.
Surgical Wounds: Used in post-surgical recovery, amniotic membrane grafts speed healing and minimize scarring across various procedures.
Orthopedics and Regenerative Medicine: Supports tissue repair in sports injuries and osteoarthritis, enhancing recovery with its regenerative properties.
How is it Used in Ophthalmology?
The amniotic membrane for eye is a cornerstone of ophthalmic treatments, addressing a range of eye diseases with amniotic membrane eye procedures. Applied as a biological bandage or amniotic membrane graft, it promotes corneal healing, reduces inflammation, and improves vision with minimal amniotic membrane eye side effects. Key conditions treated include:
- Dry Eye Disease: Amniotic membrane for dry eye relieves chronic dryness and discomfort by restoring the ocular surface.
- Corneal Ulcers: Amniotic membrane eye treatment accelerates the repair of ulcerated corneas, preventing vision loss.
- Pterygium: Used in pterygium surgery, amniotic membrane grafts reduce recurrence and inflammation.
- Persistent Epithelial Defects: Supports healing of stubborn corneal defects, enhancing patient outcomes.
Amniotic Membrane Market Growth and Trends
The amniotic membrane market was valued at USD 1 billion in 2024 and is projected to reach approximately USD 2 billion by 2032, growing at a CAGR of 7.33% from 2025 to 2032. This significant growth is driven by rising cases of chronic wounds, such as burns, trauma, and diabetic foot ulcers (DFUs), with approximately 1.6 million people in the U.S. living with DFUs in 2023, per the National Institute of Health. Amniotic membrane grafts, rich in growth factors and anti-inflammatory agents, promote tissue regeneration and healing, making them highly effective for these conditions.
The surge in ophthalmic disorders, including amniotic membrane for dry eye (affecting ~22.5 million in the U.S. in 2023) and neurotrophic keratopathy (~32,000 cases), further fuels demand for amniotic membrane eye treatments, which provide lubrication and support corneal repair with minimal amniotic membrane eye side effects.

Robust product development by key players, such as Celularity Inc. and Verséa Health’s 2023 commercialization of BIOVANCE® and BIOVANCE® 3L Ocular for amniotic membrane eye procedures, drives innovation and adoption. Growing awareness of the membrane’s regenerative properties among healthcare providers and patients globally enhances market expansion.
North America holds the largest market share in 2024, driven by advanced healthcare infrastructure, a high prevalence of chronic wounds and ophthalmic disorders, and strong product development by companies like BioTissue, Corza Ophthalmology, Integra LifeSciences, Merakris Therapeutics, Seed Biotech, Atlas Ocular, BioLab Holdings, Orthofix Medical, Labtician Ophthalmics, Smith & Nephew, Organogenesis, Celularity, Tides Medical, StimLabs, MIMEDX Group, NuVision Biotherapies, Next Biosciences, Sanara MedTech, Axogen Corporation, Dynamic Medical Services DBA Acesso Biologics, and others. The region’s dominance is bolstered by the effectiveness of amniotic membrane for eye treatments in addressing conditions like corneal ulcers and dry eye, supported by innovations such as cryopreserved amniotic membrane grafts for ocular surface restoration.
Innovations and Advancements in Amniotic Membrane Products
The amniotic membrane market is evolving with pioneering products and research, transforming treatments across medical fields. Key advancements include:
- Celularity’s DDHAM-3L Research (June 2025): A study in Regenerative Engineering and Translational Medicine showed the tri-layer decellularized, dehydrated amniotic membrane for eye supports limbal stem cell proliferation for treating limbal stem cell deficiency and ocular surface disorders. This research advances amniotic membrane eye treatment, opening new possibilities for ocular therapies.
- BioTissue’s Clarix® 1K (April 2025): An ultra-thick cryopreserved amniotic membrane graft used in robotic ureteral reconstruction, with a study of 17 patients showing 88% symptomatic improvement and 76% radiographic improvement, offering a safe alternative to autologous grafts. This advancement expands the amniotic membrane market into urology, showcasing its versatility.
- Organogenesis’s Manufacturing Expansion (November 2024): A new 122,000-square-foot biomanufacturing facility in Smithfield, Rhode Island, to boost production of advanced wound care and surgical products. This expansion ensures greater availability of amniotic membrane grafts, fueling market growth.
- Merakris’s Dermacyte AC Matrix (October 2024): A lyophilized amnion-chorion membrane allograft launched at the Fall Symposium for Advanced Wound Care, featuring enhanced protein retention for deep tissue defect protection. This product strengthens the amniotic membrane market by improving outcomes in complex wound care.
- BioTissue’s CAM360 AG (June 2024): A hydrated, shelf-stable cryopreserved amniotic membrane for eye treatment, designed for mild to moderate amniotic membrane for dry eye and ocular surface conditions, with adhesive properties ensuring comfort in amniotic membrane eye procedures and minimal amniotic membrane eye side effects. This innovation simplifies amniotic membrane eye treatment, offering a convenient solution for patients and clinicians.
- Axogen’s Avive+ Soft Tissue Matrix™ (June 2024): A resorbable multi-layer amniotic membrane graft for peripheral nerve healing, compliant with FDA regulations as a 361 human tissue product. This development enhances nerve repair solutions, broadening the amniotic membrane market’s impact.
These innovations highlight the dynamic growth of the amniotic membrane market, with advancements like CAM360 AG and DDHAM-3L enhancing amniotic membrane eye treatments and products like Dermacyte AC Matrix and Avive+ expanding applications in wound care and nerve repair. As key players invest in research and manufacturing, the market is poised to deliver cutting-edge amniotic membrane grafts, improving patient outcomes across diverse medical fields.
Challenges in the Amniotic Membrane Market
The amniotic membrane market faces several hurdles that could impact its growth and adoption. Key challenges include:
- Stringent Regulatory Requirements: Regulatory bodies like the FDA and EMA impose rigorous guidelines for the collection, processing, and distribution of amniotic membrane grafts, leading to lengthy approval processes and increased costs. For instance, in October 2022, Vitti Labs received an FDA warning letter for non-compliance in amniotic membrane product manufacturing, highlighting the complexity of regulatory adherence. These strict regulations ensure safety but can delay product launches and limit market entry for smaller players.
- High Production and Processing Costs: Procuring and preparing amniotic membrane for eye treatments and other applications is expensive due to donor screening, testing, and advanced preservation techniques like cryopreservation, making amniotic membrane eye procedures less accessible for smaller healthcare facilities. Cost barriers may restrict widespread adoption, particularly in resource-constrained settings.
- Variability in Product Quality: Inconsistent processing methods and sourcing of amniotic membranes can lead to variations in efficacy and safety, impacting outcomes in amniotic membrane eye treatments and wound care applications. Lack of standardized protocols risks reduced trust among healthcare providers, hindering market growth.
- Ethical and Infection Concerns: Ethical issues surrounding the use of human-derived tissues and the potential risk of disease transmission in amniotic membrane grafts may deter some patients and providers, despite the low amniotic membrane eye side effects. Addressing these concerns is critical to building confidence in amniotic membrane therapies.
Navigating regulatory complexities, high costs, quality variability, and ethical concerns poses significant challenges for the amniotic membrane market. Overcoming these hurdles through standardized processes and increased transparency will be essential to ensure broader adoption of amniotic membrane eye treatments and other applications.
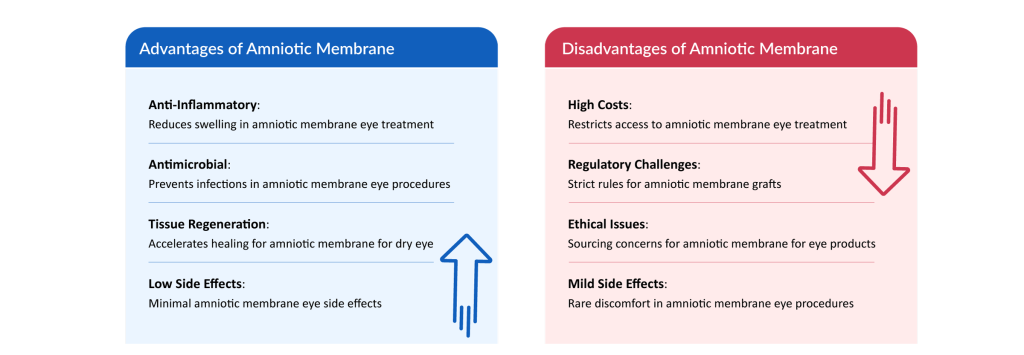
While the amniotic membrane offers distinct advantages in healing and regenerative care, it also presents notable limitations, as outlined above.
Future Outlook for the Amniotic Membrane Market
The amniotic membrane market is entering a promising era, powered by groundbreaking innovations and a rapidly widening range of clinical applications. Amniotic membrane eye treatment is advancing rapidly, with clinicians harnessing its regenerative properties to treat complex ocular surface disorders and enhance recovery in minimally invasive procedures.
A major breakthrough in amniotic membrane eye procedures has been the development of novel preservation techniques. NuVision Biotherapies’ Omnigen, a vacuum-dried amniotic membrane product exemplifies this progress. It offers room-temperature stability and quick rehydration, greatly improving accessibility for amniotic membrane eye procedures in outpatient or ambulatory care settings. These innovations are expected to streamline workflows and broaden the use of amniotic membrane eye treatment in clinical practice.
Meanwhile, researchers are expanding the therapeutic boundaries of amniotic membrane for eye applications. Beyond traditional uses, it’s now being studied for conditions like limbal stem cell deficiency and in fields such as urology and cardiovascular surgery. This diversification increases the therapeutic footprint of amniotic membrane grafts, allowing them to serve both specialized and broader surgical needs.
Growth is also accelerating in emerging markets such as China and India. Rising healthcare investment and the growing burden of conditions like chronic wounds and dry eye syndrome are driving increased demand for amniotic membrane for dry eye solutions. These regions present valuable opportunities for companies looking to scale access and enhance local treatment capabilities.
Collaborations continue to drive R&D momentum. A key example is Celularity’s DDHAM-3L study (June 2025), which is exploring advanced amniotic membrane eye applications in regenerative therapies. Such partnerships are essential in improving product efficacy, understanding amniotic membrane eye side effects, and building clinical trust.
In summary, the future of the amniotic membrane market is being shaped by innovation, therapeutic versatility, and global expansion. With a growing focus on amniotic membrane eye applications—from dry eye treatment to stem cell repair, the field is set to transform how clinicians deliver regenerative care across specialties.
Conclusion
The amniotic membrane market is on a transformative path, driven by its regenerative properties and versatile applications in amniotic membrane eye treatments, wound care, and beyond. Innovations like BioTissue’s CAM360 AG and Celularity’s DDHAM-3L are improving outcomes for conditions like amniotic membrane for dry eye and limbal stem cell deficiency, while expanded manufacturing by companies like Organogenesis ensures greater availability of amniotic membrane grafts. Despite challenges like regulatory hurdles and high costs, the market’s future is bright, with growing demand in emerging regions and new therapeutic areas.
As awareness and technological advancements continue to rise, amniotic membrane eye procedures and other treatments will play a pivotal role in advancing regenerative medicine, offering hope for improved patient outcomes worldwide.

Downloads
Article in PDF
Recent Articles
- Teleflex Receives FDA 510(k) Clearance to Expand Use of QuikClot Control+™; Edwards Wins FDA Nod ...
- BD’s Advanced Ultrasound Technology for IV Insertions; Compal Electronics Launched New RFA System...
- Myopia Medical Devices That Are Transforming Vision Care
- Sarepta Therapeutics Pauses ELEVIDYS Shipments in U.S. After FDA Intervention Over Patient Deaths...
- Familial Amyloid Polyneuropathy – An uncommon medical condition




