Current Pheochromocytomas and Paragangliomas Treatment Paradigms: Challenges and Opportunities
Jul 07, 2025
Table of Contents
The metastatic pheochromocytomas and paragangliomas treatment landscape has seen notable progress alongside ongoing challenges. Although curative therapies are still lacking, patients benefit from a multidisciplinary care strategy involving debulking surgery, chemotherapy, tyrosine kinase inhibitors, immunotherapy, and radionuclide therapy with 177Lu-DOTATATE.
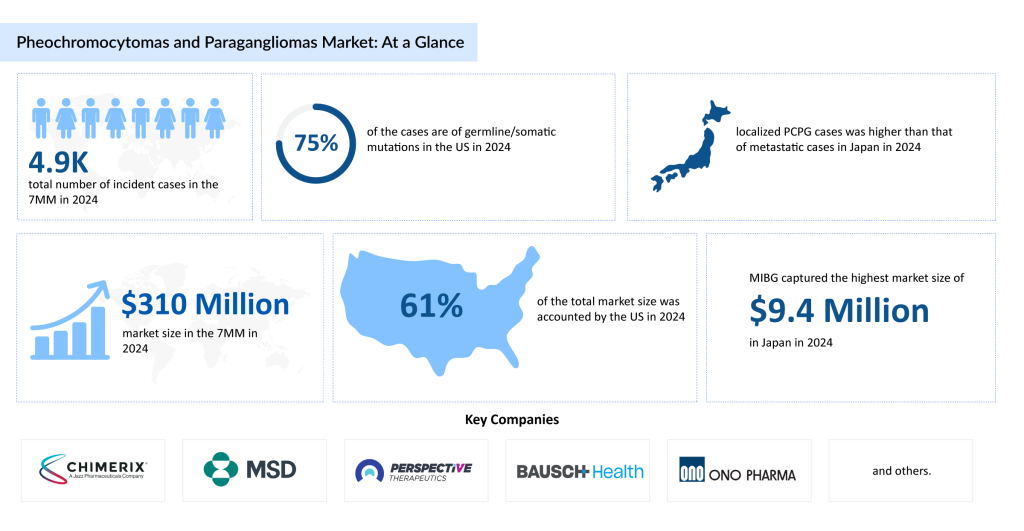
Around 10% of pheochromocytomas and 40% of paragangliomas are considered malignant. Germline genetic mutations are detected in approximately 30% of patients, with alterations in SDHx genes associated with a higher risk of malignancy. In 2024, the number of newly diagnosed PCPG cases across the 7MM reached approximately 4,900, and this figure is expected to rise over the forecast period. In the US specifically, germline or somatic mutations have been reported in nearly 75% of cases in 2024.
Downloads
Click Here To Get the Article in PDF
Recent Articles
Earlier, a significant number of PCPG cases remained undiagnosed during a patient’s lifetime and were only identified during autopsies. However, in recent years, the widespread use of imaging techniques in clinical practice has greatly increased. These advancements in diagnostic methods have likely contributed to a higher detection rate of PPGL during a person’s lifetime. As a result, the incidence of PPGL has risen over the past two decades.
For functioning metastatic PCPG, initial management typically involves alpha-blockade, followed by beta-blockers, calcium channel blockers, and metyrosine as needed. In cases of localized PCPG, radical surgical resection remains the primary treatment approach and the only potentially curative option.
DEMSER (metyrosine) received FDA approval in the United States in 1979 for the treatment of pheochromocytoma. This medication is used to prepare patients for surgery, manage those who are not candidates for surgery, and provide long-term treatment for malignant pheochromocytoma. Managing metastatic pheochromocytomas and paragangliomas remains difficult, as few standardized systemic therapies have been widely adopted outside of clinical trial settings.
In July 2018, the FDA approved AZEDRA for PCPG treatment, following results from Study IB12B, which involved patients with iobenguane scan-positive, unresectable, locally advanced, or metastatic PCPG. However, on August 15, 2023, Lantheus announced plans to halt AZEDRA production and close its Somerset, New Jersey manufacturing facility. This decision, affecting the only FDA-approved therapy for PCPG, was attributed to low commercial demand, with manufacturing scheduled to continue only through the first quarter of 2024.
WELIREG Approval Marks a Significant Step Forward in PCPG Treatment
Six years after acquiring Peloton Therapeutics, Merck is advancing the key asset from that deal rather than letting it sit idle. In May 2025, the FDA granted WELIREG (belzutifan) its third approved use, clearing the HIF-2α inhibitor for the treatment of patients with pheochromocytoma or paraganglioma, rare tumors found in the endocrine system.
This new approval applies to patients aged 12 and older with locally advanced, inoperable, or metastatic PPGL. With this, WELIREG becomes the first oral therapy available for advanced forms of PPGL, according to Merck.
WELIREG is a first-in-class, highly selective HIF-2α inhibitor. It is also approved for patients with von Hippel-Lindau (VHL) disease who require treatment for certain tumors: renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, and pancreatic neuroendocrine tumors (pNET) that are not immediately needing surgery, as well as advanced RCC following prior PD-1/PD-L1 and VEGF-targeted therapies.
The latest FDA approval was supported by data from the LITESPARK-015 study, a single-arm clinical trial. The primary measure of success was objective response rate (ORR), evaluated in 72 patients (Cohort A1) with measurable PPGL, confirmed by blinded independent central review (BICR) using RECIST v1.1 criteria. All participants had histologically confirmed locally advanced or metastatic PPGL that could not be removed surgically or cured. Patients enrolled had stable blood pressure (below 150/90 mm Hg in adults and below 135/85 mm Hg in adolescents) without recent adjustments in their antihypertensive medications.
Those with carcinomatous meningitis were excluded from the trial. Participants received a daily 120 mg dose of WELIREG until their disease worsened or unacceptable side effects developed. The main efficacy outcome was ORR as assessed by BICR, with secondary measures including response duration and time to first response.
WELIREG’s labeling includes a boxed warning about the risk of fetal harm if taken during pregnancy. Physicians are instructed to verify pregnancy status before starting therapy and to counsel patients on using reliable non-hormonal contraception, as WELIREG may reduce the effectiveness of hormonal birth control. Other significant risks include severe anemia, which might require blood transfusions, and serious hypoxia, which could lead to treatment discontinuation, oxygen therapy, or hospitalization. Ongoing monitoring for anemia is recommended before and during treatment.
New Frontiers in Targeted PCPG Treatment: Clinical Advances with SSTR2, DRD2, and ClpP Agonists
Several PCPG drugs are currently in development, including ONC201 (by Jazz Pharmaceuticals/Chimerix), [212Pb]VMT-α-NET (by Perspective Therapeutics), and LUTATHERA (Lutetium [177Lu] oxodotreotide/dotatate) (Novartis), among others.
ONC201, also known as dordaviprone, is a first-in-class imipridone small molecule that targets the dopamine receptor D2 (DRD2) and the mitochondrial protease ClpP. In a Phase II open-label investigator-initiated study at the Cleveland Clinic, ONC201 was evaluated in 30 patients with rare neuroendocrine tumors. Interim results demonstrated a 50% overall response rate (ORR) in patients with dopamine-secreting paragangliomas located outside the brain. These findings were presented at the American Society of Clinical Oncology (ASCO) Annual Meeting in 2021 and subsequently published in Clinical Cancer Research in 2022.
[212Pb]VMT-α-NET is an investigational Targeted Alpha-particle Therapy (TAT) radiopharmaceutical designed to treat and diagnose neuroendocrine tumors expressing somatostatin receptor subtype 2 (SSTR2), a rare and challenging cancer type. Perspective Therapeutics is running a multicenter, open-label Phase I/IIa trial (NCT05636618) involving dose-escalation and dose-expansion cohorts in patients with unresectable or metastatic SSTR2-positive neuroendocrine tumors who have not undergone prior peptide receptor radionuclide therapy (PRRT). Based on promising preclinical results, the U.S. FDA granted Fast Track Designation (FTD) for VMT-α-NET in SSTR2-positive NETs regardless of previous treatment history. By the end of February 2025, 30 patients had been enrolled in Cohort 2 of this trial, while regulatory alignment for initiating Cohort 3 was still pending.
In June 2025, Perspective Therapeutics announced that it had secured FDA approval to begin enrollment for Cohort 3 of its ongoing Phase I/IIa trial in patients with unresectable or metastatic SSTR2-positive NETs who have not previously received radiopharmaceutical therapies. Additionally, updated interim results from this trial were presented at the 2025 ASCO Annual Meeting, held from May 30 to June 3 in Chicago, Illinois.
The anticipated launch of these emerging therapies are poised to transform the PCPG treatment market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the PCPG market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Expected Roadblock in PCPG Treatment
One of the key expected roadblocks in the treatment of pheochromocytomas and paragangliomas is the limited availability of targeted therapies, despite advances in understanding the molecular drivers of the disease. The rarity and heterogeneity of PCPG create challenges in conducting large-scale clinical trials, making it difficult to generate robust efficacy and safety data for emerging drugs. Additionally, delayed diagnosis due to non-specific symptoms and variability in disease presentation often leads to advanced-stage detection, further complicating treatment outcomes. Access to novel therapies may also be hindered by high costs, limited reimbursement policies, and the need for specialized care centers capable of managing these complex tumors. Lastly, resistance to existing treatments and the lack of validated biomarkers for treatment selection and monitoring remain significant hurdles to improving long-term patient outcomes.
Future PCPG Treatment Outlook Looks Promising
The future outlook for the treatment of pheochromocytomas and paragangliomas appears highly promising, driven by advances in molecular research, targeted therapies, and immunotherapy. New drug candidates such as HIF-2α inhibitors have shown encouraging results, especially in patients with underlying genetic mutations like VHL syndrome.
As per DelveInsight analysis, the total market size of PCPG in the 7MM was around USD 310 million in 2024. This is estimated to increase by 2034. In 2024, MIBG captured the highest market size of PCPG by therapies of approximately USD 10 million in Japan.
Additionally, radiopharmaceutical therapies, including peptide receptor radionuclide therapy (PRRT) and novel alpha-emitting radioligands, are expanding the therapeutic arsenal, offering targeted approaches with potentially fewer systemic side effects. Continued focus on biomarker discovery and next-generation sequencing is also helping to refine treatment selection, allowing for more personalized and effective management of PCPG patients.
Moreover, collaborations between academic centers, pharmaceutical companies, and patient advocacy groups are driving the acceleration of clinical trials and regulatory approvals in this rare cancer space. The integration of artificial intelligence and machine learning in PCPG research is expected to further enhance diagnostic accuracy, predict treatment response, and optimize clinical trial designs.
As treatment paradigms shift toward combination regimens and earlier intervention strategies, patients are likely to benefit from improved survival rates and better quality of life. Overall, the therapeutic landscape for PCPG is transitioning from symptomatic management and surgery toward precision medicine approaches that offer hope for long-term disease control.

Downloads
Article in PDF




