Leo’s ANZUPGO Cream: First FDA-Approved Treatment for Adults with Moderate-to-Severe Chronic Hand Eczema
Sep 26, 2025
Chronic hand eczema is more than just dry, itchy skin—it’s a persistent condition that affects up to 10% of adults worldwide, often interfering with daily life and work. Chronic hand eczema is characterized by either a persistent or relapsing course of hand eczema, or by eczema that does not respond to standard treatments with emollients and topical corticosteroids for over three months, or by the recurrence of symptoms two or more times within a year despite treatment. Females generally experience an earlier onset of CHE and show a higher overall CHE prevalence compared to males. The condition typically develops around the age of 12.
While CHE is not limited to individuals with atopic dermatitis, having atopic dermatitis increases the risk of developing the condition. The management of CHE often involves a stepwise approach, beginning with the avoidance of triggers and the frequent use of barrier creams, and may progress to include topical therapies, physical treatments such as UV light, and systemic options, depending on the severity of the disease. Although there are multiple treatment options for CHE, their effectiveness is often limited.
ANZUPGO Cream Marks FDA Milestone in Hand Eczema Care
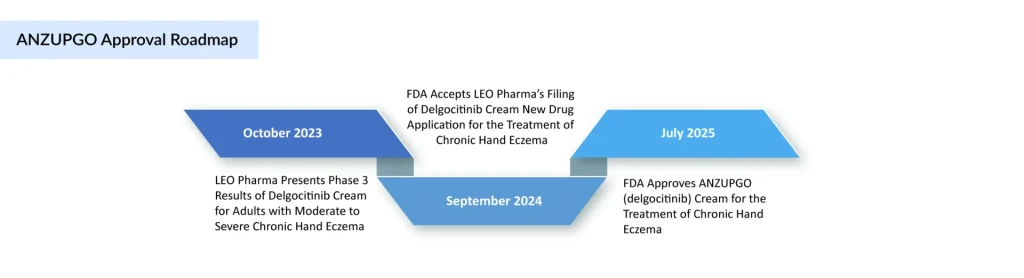
Danish dermatology company Leo Pharma has received FDA approval for its JAK inhibitor cream, ANZUPGO (delgocitinib), making it the first therapy in the US specifically approved for the treatment of chronic hand eczema.
Downloads
Click Here To Get the Article in PDF
The ANZUPGO approval received in July 2025 applies to adults with moderate-to-severe CHE who have not responded to, or cannot use, topical corticosteroids. Last September, ANZUPGO became the first topical therapy for CHE to gain approval in Europe.
Dermatologists have welcomed the approval as a much-needed option for a condition that affects 1 in 10 adults globally but is often overlooked. A recent survey of nearly 200 dermatologists, conducted by Ipsos on behalf of Leo, found that more than half expressed frustration over the lack of progress in treating CHE.
Leo also highlighted that ANZUPGO is the first topical pan-JAK inhibitor to reach the U.S. market. Pan-JAK inhibitors target multiple JAK enzymes, whereas selective JAK inhibitors focus on specific ones. The only other topical JAK inhibitor approved in the US is Incyte’s OPZELURA, indicated for eczema and vitiligo.
By blocking the JAK-STAT pathway, ANZUPGO inhibits JAK1, JAK2, JAK3, and tyrosine kinase 2, reducing the inflammatory responses that drive CHE flares. Leo acquired delgocitinib in 2014 from Japan Tobacco, which continues to market the drug in Japan, where it has been available for five years.
The FDA approval was supported by two pivotal Phase III trials, DELTA 1 and DELTA 2, which evaluated 960 adults with moderate-to-severe CHE. Both studies met their primary and key secondary endpoints. At Week 16, nearly half of patients treated with ANZUPGO achieved a ≥4-point reduction in both pain and itch, compared to roughly a quarter in the cream vehicle groups (p<0.0001).
Leo Pharma is actively working to make ANZUPGO accessible in the US as quickly as possible, emphasizing its commitment to broad and affordable patient access.
Emerging Competitors to Leo’s ANZUPGO
Other companies, such as Regeneron and Sanofi, are also seeking approval for CHE, with their highly successful drug DUPIXENT leading the way. DUPIXENT (dupilumab) is a targeted biologic treatment that has shown promising results for chronic hand eczema, especially in cases that are moderate to severe or resistant to conventional therapies. By inhibiting interleukins IL-4 and IL-13, which play key roles in the inflammatory process, Dupixent helps reduce symptoms such as redness, itching, and skin thickening, improving overall skin condition and quality of life for patients.
Studies, including systematic reviews and meta-analyses, indicate that approximately 80% of patients with CHE experience partial or complete resolution of symptoms within 4-16 weeks of starting DUPIXENT, with sustained benefits often extending beyond treatment periods.
Meanwhile, Asana Biosciences’ ASN002 is a potent oral inhibitor targeting both Janus Kinase (JAK) and spleen tyrosine kinase (SYK). These kinases play key roles in cytokine production and signaling and have been linked to the development of various lymphomas, solid tumors, myeloproliferative disorders, and inflammatory conditions. Phase IIb chronic hand eczema clinical trials evaluating ASN002 have been completed. Additionally, the FDA has granted ASN002 Fast Track designation for the treatment of moderate to severe chronic hand eczema.
Future Outlook of Chronic Hand Eczema Treatment Space
The approval of ANZUPGO cream by the FDA in July 2025 marks a significant milestone in the treatment of chronic hand eczema. As the first and only FDA-approved topical therapy specifically for moderate-to-severe CHE in adults, ANZUPGO addresses a longstanding unmet need in dermatology.
The approval of ANZUPGO is expected to transform the treatment landscape for CHE. Clinical trials have demonstrated significant improvements in skin clearance, pain relief, and overall quality of life for patients using ANZUPGO. This advancement provides healthcare providers with a targeted, non-steroidal option for patients who have not responded adequately to traditional treatments. With its availability in the US, European Union, United Kingdom, Switzerland, and the United Arab Emirates, ANZUPGO is poised to become a cornerstone in the management of CHE.
Apart from Leo, other companies are also working with their lead assets to enter the CHE treatment space. Oral therapies with dual-target mechanisms, such as JAK/SYK inhibitors, are being explored to provide systemic control for patients with refractory disease. The integration of these targeted treatments into clinical practice may also reduce reliance on long-term corticosteroids, minimizing side effects and improving patient adherence.
Looking ahead, digital health solutions and biomarker-driven approaches are likely to transform the CHE landscape further. Non-invasive diagnostic tools, predictive biomarkers for disease severity, and treatment response monitoring could enable personalized treatment regimens, thereby optimizing outcomes and reducing the need for trial-and-error therapy. Combined with ongoing innovation in topical formulations and systemic therapies, the CHE treatment space is poised for a shift toward more effective, tailored, and patient-centric care. Overall, the future outlook suggests a change from symptomatic management to disease-modifying strategies, which can substantially improve long-term outcomes for patients.

Downloads
Article in PDF



