Spondylolisthesis Medical Devices: Advancing Care Through Innovation
Dec 31, 2025
Table of Contents
Spondylolisthesis, a formidable spinal condition characterized by the slippage of one vertebra over the one below it, represents a significant global health burden. It is a source of chronic, debilitating low back pain, neurological deficits, and reduced quality of life for millions. Historically, the management of spondylolisthesis, which affects an estimated 6-8% of the adult population, with a higher prevalence of degenerative cases in women and older adults, has evolved from conservative care to complex, high-stakes surgical interventions.
The core challenge in treating symptomatic spondylolisthesis lies in achieving three critical goals: decompressing the compromised neural structures, stabilizing the unstable vertebral segment, and restoring proper spinal alignment. The limitations of traditional open surgeries, such as extensive soft tissue damage, prolonged recovery times, and the risk of adjacent segment degeneration (ASD) following rigid fusion, have spurred a fervent wave of innovation in the medical device industry. This innovation has birthed a new generation of sophisticated devices and procedural technologies designed to make spinal surgery safer, more precise, and less invasive, ultimately advancing patient care.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Seno Medical Secures EU MDR CE Mark for Next-Generation Imagio® Imaging System; Spine Innovation ...
- Establishment Labs Gains FDA Approval for Motiva Implants; Surmodics Receives 510(k) Clearance fo...
- SetPoint Medical Earns FDA Nod for First-of-Its-Kind Neuroimmune Modulation Therapy in RA; Body V...
- Neuspera Medical’s Nuvella System; Abbott Presented the Data for FreeStyle Libre 2 System; ZEISS ...
- Inspira™ Approval of INSPIRA™ ART100 System; Oticon Medical’s Sentio™ System Received Regul...
Understanding the Need for Advanced Devices
The impetus for technological advancement stems directly from the anatomical and biomechanical complexities of spondylolisthesis. The condition is broadly classified into several types (e.g., dysplastic, isthmic, degenerative), with degenerative spondylolisthesis, the most common, often presenting with an intact pars interarticularis but instability due to facet joint arthritis and disc degeneration. Isthmic spondylolisthesis, caused by a defect in the pars, is more common in younger, active patients.
Regardless of the type, the slippage leads to spinal canal and neural foramen stenosis, which compresses the nerve roots and causes symptoms such as neurogenic claudication and radicular pain. Traditional open decompression and fusion procedures, while effective, often require significant exposure, leading to major blood loss and muscle damage. Advanced medical devices address these issues by:
Minimally Invasive Access: Allowing surgeons to operate through smaller incisions, preserving muscle, and reducing post-operative pain and hospital stay.
Enhanced Fixation: Providing superior biomechanical stability to the spine, lowering the risk of hardware failure and pseudarthrosis.
Motion Preservation: Offering non-fusion alternatives for select patients, which aims to mitigate the long-term risk of ASD.
Precision and Safety: Integrating imaging and navigation to increase the accuracy of implant placement and reduce the risk of neurological injury.
Key Categories of Medical Devices for Spondylolisthesis
The device landscape for spondylolisthesis is diverse, encompassing technologies for rigid fixation, dynamic stabilization, and surgical support.
Spinal Fusion Systems
The gold standard for treating symptomatic, unstable spondylolisthesis that has not responded to conservative care is Spinal Fusion, which aims to weld two or more adjacent vertebrae into a single, stable bone mass. The current medical device market relies heavily on modern fusion systems to achieve this goal. At the core of these systems are Pedicle Screw and Rod Systems, which provide rigid internal fixation. Pedicle screws function as secure anchors, inserted into the vertebral pedicles, and are connected by longitudinal rods to stabilize the segment. Innovations in this area continually refine their biomechanical efficacy and surgical approach. These include the development of Polyaxial/Uniplanar Screws, which offer varying degrees of angulation to facilitate easier and more efficient rod placement. A more specialized innovation is the Cortical Bone Trajectory (CBT) Screws technique, which uses a medial-to-lateral, caudal-to-cranial insertion path. This trajectory is advantageous as it allows for smaller incisions, potentially reduces muscle dissection, and engages the denser cortical bone, thus maintaining strong fixation with a less invasive profile.
Interbody Fusion Cages: Interbody Fusion Cages are essential components in the treatment of spondylolisthesis, placed in the disc space following removal of the diseased disc (discectomy). These devices serve multiple critical functions: they restore the proper disc height, which indirectly decompresses the neural elements, and they provide a substantial surface area to pack with bone graft, thereby promoting successful fusion. Modern interbody cages are undergoing rapid evolution across several key areas. Regarding Materials, there has been a significant shift from older materials such as solid titanium and cobalt-chrome toward radiolucent Polyether Ether Ketone (PEEK) and, increasingly, highly porous 3D-printed titanium, designed to promote superior bone ingrowth and on-growth.
A significant technological advancement is Expandable Cages: these devices can be inserted in a collapsed or minimal-profile configuration through a small incision, a key feature of Minimally Invasive Spine Surgery (MISS), and then expanded in situ within the disc space to achieve optimal distraction, fit, and restoration of lordosis. Furthermore, these cages are employed in various surgical Approaches, including the traditional Posterior Lumbar Interbody Fusion (PLIF) and Transforaminal Lumbar Interbody Fusion (TLIF) (which has its own MIS variant, MIS-TLIF), as well as the Anterior Lumbar Interbody Fusion (ALIF), and the increasingly popular Lateral Lumbar Interbody Fusion (LLIF).
Minimally Invasive Spine Surgery (MISS) Devices
MISS is rapidly replacing traditional open surgery for many spondylolisthesis cases, driven by a preference for reduced trauma, shorter hospital stays, and faster recovery. The device technology enabling MISS is characterized by specialized retraction and navigation. As per DelveInsight analysis, the global minimally invasive surgical devices market is expected to increase from USD 31 billion in 2024 to USD 53 billion by 2032, reflecting strong and sustained growth. The global minimally invasive surgical devices market is projected to grow at a CAGR of 7.13% from 2025 to 2032.
The rapid adoption of Minimally Invasive Spine Surgery (MISS) for spondylolisthesis is fueled by the desire for reduced surgical trauma, shorter hospital stays, and quicker patient recovery. The specialized device technology central to MISS relies on two primary innovations: specialized retraction and precise percutaneous fixation. Tubular Retractors form the core of the retraction system; these devices allow surgeons to access the spine through small, working corridors by sequentially dilating, rather than cutting, the soft tissue. They are essential enablers for complex MISS procedures such as MIS-TLIF and microdiscectomy combined with fixation. Complementing this, Percutaneous Fixation Systems are utilized, often in conjunction with tubular retractors. These devices permit the insertion of pedicle screws and rods through small stab incisions, thereby bypassing extensive open exposure and significantly reducing iatrogenic injury to the critical paraspinal musculature.
Dynamic Stabilization Devices
These devices aim to bridge the gap between rigid fusion and nonoperative care. They are designed to stabilize the compromised segment while preserving a controlled range of motion and reducing load on the intervertebral disc and facets, theoretically mitigating ASD risk.
Posterior Dynamic Stabilization (PDS) Systems: These use pedicle screws connected by a flexible or semi-rigid connecting element, such as a polymer cord or an articulated joint, instead of a rigid metal rod. The Total Posterior Spine (TOPS) System, for example, is an artificial facet replacement device approved as an alternative to fusion for grade I degenerative spondylolisthesis, aiming to restore and maintain physiological movement.
Interspinous Spacers
These devices are Interspinous Spacers, which represent a less invasive, non-fusion treatment option implanted directly between the spinous processes—the bony prominences located at the back of the vertebrae. Their Mechanism of Action is primarily biomechanical: they restrict spinal extension, the specific movement that often aggravates pain associated with spinal stenosis and degenerative spondylolisthesis. By effectively “propping open” the posterior aspect of the spinal segment, they indirectly decompress the neural elements, offering significant relief, particularly for patients suffering from neurogenic claudication. Their Indication for use is typically limited to carefully selected patients presenting with mild to moderate spinal stenosis, frequently accompanied by Grade I degenerative spondylolisthesis, who have not responded adequately to conservative treatments. Consequently, Interspinous Spacers are positioned as a less-invasive alternative to more extensive procedures such as formal decompression and fusion.
Neuromonitoring and Navigation Technologies
These technologies do not directly treat the pathology but are crucial adjuncts that enhance the safety and precision of the surgical procedure. Critical to the safety and precision of complex spondylolisthesis surgery are enabling technologies like Intraoperative Neuromonitoring (IONM) and advanced imaging systems. IONM involves the real-time electrophysiological assessment of the spinal cord and nerve roots during the procedure, utilizing modalities such as Somatosensory Evoked Potentials (SSEPs), Motor Evoked Potentials (MEPs), and Electromyography (EMG).
This continuous, real-time feedback is invaluable during high-risk maneuvers such as reducing high-grade slips and pedicle screw placement, serving as an essential early warning system for potential nerve damage. Complementing this protection, Surgical Navigation and Robotics systems integrate 3D fluoroscopy or intraoperative CT with sophisticated software to create a real-time “GPS” map of the patient’s spine. Navigation allows surgeons to accurately plan and execute pedicle screw placement with millimeter-level precision, significantly improving screw accuracy, especially in cases with distorted or complex anatomy. This technology also helps reduce operative time and radiation exposure compared to conventional fluoroscopy. Furthermore, Robotics platforms provide an additional layer of control, offering haptic feedback and guided assistance for high-precision tasks such as drilling and screw insertion, thereby further minimizing the risk of human error.
Advantages and Disadvantages of Spondylolisthesis Medical Devices
The primary advantages across the spectrum of spondylolisthesis devices center on stability, motion preservation, and reduced invasiveness. Spinal Fusion Systems offer the most critical benefit: proven long-term stability and pain relief, leading to a high rate of successful bony fusion (arthrodesis). Minimally Invasive Spine Surgery (MISS) Devices provide substantial benefits for patients, including reduced blood loss, shorter hospital stays, less soft tissue damage, and a faster overall recovery. Dynamic Stabilization devices offer the unique advantage of preserving some controlled spinal motion, which, theoretically, helps mitigate the long-term risk of Adjacent Segment Degeneration (ASD) above or below the treated segment. The least invasive option, Interspinous Spacers, delivers fast, often immediate, symptom relief, particularly for neurogenic claudication, and is a reversible procedure. Lastly, sophisticated tools such as Neuromonitoring and Navigation significantly improve surgical accuracy and provide real-time neurological protection, thereby drastically reducing the risk of malpositioned implants and intraoperative nerve injury.
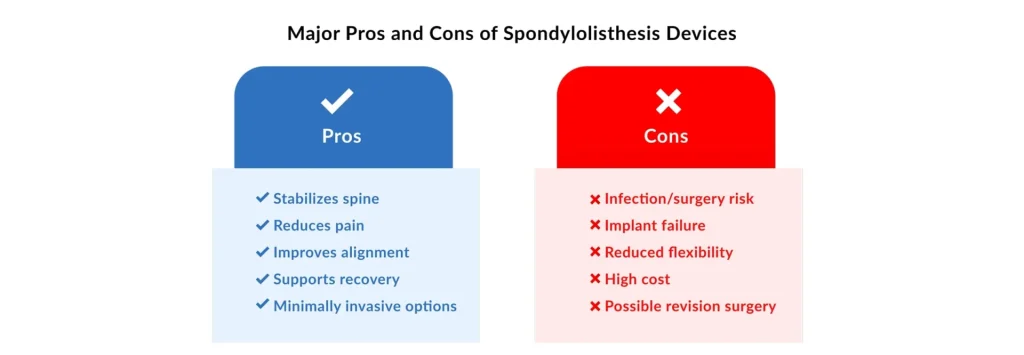
Despite their clinical benefits, these devices carry specific disadvantages. For Spinal Fusion Systems, the major drawback is the unavoidable loss of motion at the fused segment, which is directly linked to the risk of Adjacent Segment Degeneration (ASD) over time. The successful use of MISS Devices is hampered by a steep learning curve for surgeons and the need for specialized, often costly equipment. Dynamic Stabilization devices have been shown in some studies to have higher re-operation rates than rigid fusion. They are prone to mechanical issues, such as screw loosening or device failure, due to continuous cyclical loading. Interspinous Spacers have limited efficacy in treating higher-grade or purely unstable slips and carry a risk of device migration or spinous process fracture. Finally, while crucial for safety, Neuromonitoring and Navigation tools entail high capital costs and require ongoing maintenance, necessitating specially trained personnel, all while not fundamentally altering the steps of the underlying surgical procedure.
Future Outlook: Where Spondylolisthesis Devices are Heading?
The future of spondylolisthesis device technology is focused on personalization, biological integration, and automation.
Biologics and Bio-Integrated Implants
The next frontier in spondylolisthesis treatment is focused on optimizing the biological environment to enhance healing and ensure faster, more robust fusion. This involves a three-pronged approach centered on advanced materials and biological agents. Firstly, Osteobiologics represent a key innovation, encompassing advanced bone graft substitutes and potent agents like bone morphogenetic proteins (BMPs). These substances are specifically designed to accelerate bone healing and significantly boost the success rate of spinal fusion (arthrodesis) by promoting osteoinduction (bone formation). Secondly, the development of Bioactive Coatings for implants is critical; these involve surface modifications or specialized coatings that directly encourage osteointegration (the structural and functional connection between the bone and the implant surface) while also being engineered to reduce the risk of surgical site infection. Finally, the concept of Resorbable Materials is gaining traction. This involves the use of advanced polymer or ceramic implants that provide the necessary temporary mechanical stability to the spine during the initial healing phase, but then gradually and safely resorb into the body, ultimately leaving behind a solid, natural fusion mass composed of the patient’s own bone.
Smart Implants and Connected Surgery
The influence of the Internet of Medical Things (IoMT) is fundamentally reshaping spine care, transitioning it toward a more connected, data-driven environment. This revolution is most evident in the development of Smart Implants: future spinal devices are being engineered to incorporate tiny, self-powered sensors that can monitor critical variables such as the progression of bone fusion, real-time spinal loading, and even the early detection of infection by measuring temperature or chemical changes. These data can then be transmitted wirelessly and securely to the surgeon, enabling continuous, objective remote patient monitoring that goes beyond the limitations of infrequent X-rays. Simultaneously, AI-Powered Pre-operative Planning is integrating artificial intelligence and machine learning to analyze vast amounts of patient imaging and clinical data with unprecedented efficiency. These algorithms can predict ideal surgical trajectories, recommend the optimal size and placement of implants, and even stratify a patient’s risk of complications, enabling surgeons to personalize the surgical strategy and achieve a new level of precision long before the first incision is made.
Market Growth
The market for spinal implants and surgical devices is poised for significant growth, reflecting the increasing prevalence of spinal disorders in an aging global population and the continued adoption of new technologies.
In conclusion, the treatment of spondylolisthesis is being fundamentally transformed by medical device innovation. From the precision of minimally invasive fusion systems and the biomechanical nuance of dynamic stabilization to the safety net provided by neuromonitoring and navigation, these advancements are shifting the paradigm toward more personalized, less morbid, and ultimately more effective patient care. The intersection of materials science, robotics, and biological engineering promises a future in which spinal stability is restored with minimal intrusion, truly advancing the standard of care for this common and debilitating condition.

Downloads
Article in PDF
Recent Articles
- Gore Secures FDA Approval for Its First Deep Venous Stent for IVC and Iliofemoral Veins; Stereota...
- SetPoint Medical Earns FDA Nod for First-of-Its-Kind Neuroimmune Modulation Therapy in RA; Body V...
- Sonomotion Secures FDA Clearance for Break Wave™ Lithotripsy System; Aidoc Earns FDA Clearance fo...
- FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s...
- Establishment Labs Gains FDA Approval for Motiva Implants; Surmodics Receives 510(k) Clearance fo...




